Abstract
Background
Corneal and conjunctival epithelial dendritic cells (DC) have an established role in vernal keratoconjunctivitis, however, their role in more prevalent forms of allergic eye disease remains unclear. This study evaluated corneal and conjunctival epithelial DC density, morphology, and distribution observed using in vivo confocal microscopy (IVCM) in allergic conjunctivitis.
Methods
In this prospective, observational study, 66 participants (mean age 36.6 ± 12.0 years, 56% female): 33 with allergic conjunctivitis and 33 controls were recruited. IVCM was performed at the corneal centre, inferior whorl, corneal periphery, corneal limbus, and temporal bulbar conjunctiva. DC were counted and their morphology was assessed as follows: largest cell body size, presence of dendrites, and presence of long and thick dendrites. Mixed model analysis (DC density) and non-parametric tests (DC morphology) were used.
Results
DC density was higher in allergic participants at all locations (p ≤ 0.01), (corneal centre median (IQR) 21.9 (8.7–50.9) cells/mm2 vs 13.1 (2.8–22.8) cells/mm2; periphery 37.5 (15.6-67.2) cells/mm2 vs 20 (9.4–32.5) cells/mm2; limbus 75 (60-120) cells/mm2 vs 58.1 (44.4–66.2) cells/mm2; conjunctiva 10 (0–54.4) cells/mm2 vs 0.6 (0–5.6) cells/mm2, but not at the inferior whorl 21.9 (6.2–34.4) cells/mm2 vs 12.5 (1.9–37.5) cells/mm2, p = 0.20. At the corneal centre, allergic participants had larger DC bodies (p = 0.02), a higher proportion of DC with dendrites (p = 0.02) and long dendrites (p = 0.003) compared to controls.
Conclusions
Corneal and conjunctival DC density was increased, and morphology altered in allergic conjunctivitis. These findings imply that the ocular surface immune response was upregulated and support an increased antigen-capture capacity of DC in allergic conjunctivitis.
Similar content being viewed by others
Introduction
Ocular allergy is an inflammatory reaction of the ocular surface resulting from a hypersensitivity reaction of the ocular adnexa to environmental allergens. Seasonal and perennial allergic conjunctivitis are most common, their incidence ranging from 6–30% [1, 2]. Whilst these milder forms of allergy result in bothersome symptoms and signs, they nevertheless pose a significant societal burden [3, 4]. Understanding the pathophysiology of allergy can optimise its diagnosis and management.
Dendritic cells (DC) are initiators of the allergic immune response [5,6,7]. DC capture and process antigens and migrate from affected tissues (e.g., cornea) to the lymph nodes and present antigens to T cells to initiate the immune response [5, 8]. In vivo confocal microscopy (IVCM) enables observation of DC in vivo. Increased DC density and altered morphology can signal an inflammatory or immune response [9]. The role of DC in ocular allergy is not well established.
DC density is increased in vernal keratoconjunctivitis (VKC) however it has not been investigated in allergic conjunctivitis [10,11,12]. In vivo studies have focused on characterising central DC density with little consideration of peripheral regions or DC morphology, both of which are important factors related to DC activation and migration. Although the primary site of ocular allergy is the conjunctiva, the cornea and conjunctiva crosstalk through release of mediators and cytokines [13,14,15]. When inflammation is present, DC are recruited from the limbus and move to the central cornea [16, 17]. Examining many locations concurrently gives better insights into topographical variations in immune activation during allergic eye disease. Alterations of DC morphology characterise the immune response and can serve as an early marker of disease [18, 19]. Corneal DC in participants with systemic allergy had longer dendrites compared to the non-allergic group, suggesting an increased antigen-capture capacity of DC at the ocular surface [20].
The reliability and reproducibility of DC measurements using IVCM must be established. We have reported inter and intra-observer repeatability of DC morphology and intra-observer of DC density [21, 22]. However, the inter-observer repeatability of DC density at various locations has not been reported. This study aimed to evaluate the topographical distribution, density, and morphology of corneal and conjunctival DC observed with IVCM in participants with allergic conjunctivitis. A secondary aim was to assess the inter-observer repeatability of DC density measurement at various locations.
Materials and methods
Study design and participants
A prospective, cross-sectional observational study was conducted. The study adhered to the Declaration of Helsinki, and participants were appraised of study procedures. Informed consent was obtained prior to enrolment in the study. The Sydney Children’s Hospitals Network Human Research Ethics Committee (HREC) (2019/ETH11844) and HREC of UNSW Sydney (HC180930) approved the study.
Participants over 18 years old were recruited. Participants with or without a prior diagnosis of allergic conjunctivitis, who had current symptoms of ocular allergy or hayfever and a positive skin prick test were assigned to the allergy group. Participants without a history or diagnosis of allergic conjunctivitis, without symptoms and a negative skin prick test were included as controls. A standard skin prick test for ten common indoor and outdoor aeroallergens of the Sydney region was conducted (Stallergenes, Antony, France, and Inmunotek, alergie e immunologia, Spain): three grass pollens, tree pollen, plant pollen, two dust mites, mould, cat, and dog dander. Participants were classified as positive if the wheal diameter was ≥3 mm for any allergen [23].
Participants were excluded if they reported use of ocular lubricants in the week prior, or current use of topical or systemic antihistamine or mast cell stabiliser or nasal corticosteroid spray or immunotherapy for aeroallergens. Other exclusion criteria included uncontrolled asthma, past anaphylactic episode, current pregnancy or breastfeeding, regular contact lens wear, ocular surface disease other than ocular allergy, Sjøgren syndrome, active intraocular inflammation, history of refractive or ocular surgery, or systemic conditions that can affect the ocular surface (e.g., diabetes, rheumatoid arthritis).
Sample size was calculated using GPower 3.1. If the controls show a mean DC density of 30 cells/mm2 at the corneal centre (SD = 35 cells/mm2) and the allergy group shows a mean density of 58 cells/mm2 and similar SD, there would be greater than 80% power to detect the mean difference with 27 participants per group (54 total), assuming alpha of 0.05 [22]. A 15% adjustment allowed for possible non-normal distribution in outcome variable, for a total 33 per group.
Symptoms and signs
The Aston University Allergy Questionnaire (AUAQ), Ocular Surface Disease Index (OSDI), and Dry Eye Questionnaire (DEQ-5) were used to record ocular surface symptoms [24,25,26]. Non-invasive tear break-up time was assessed using a tearscope (Keeler Ltd, United Kingdom). The BHVI grading system was used to record redness [27]. Corneal epithelial disorder, bulbar conjunctival chemosis, palpebral conjunctival papillae, and follicles were graded using the Japanese grading scale [28]. The Oxford grading scale was used to grade staining [29].
DC density and morphology
The HRT III confocal microscope with Rostock Corneal Module (Heidelberg Engineering GmbH, Heidelberg, Germany) was used to assess corneal and conjunctival DC [22]. Five locations of the corneal subbasal epithelium and conjunctival epithelium including the corneal anatomical centre, inferior whorl, peripheral cornea (temporal, 1 mm inside limbus), limbal cornea (temporal), and bulbar conjunctiva (temporal, 2–3 mm from the limbus) of the right eye were scanned, in that order. The corneal subbasal epithelium was detected at 35–70 µm and conjunctival epithelium at 5–20 µm depth. Topical anaesthetic (oxybuprocaine 0.4%, Minims, Bausch & Lomb UK) and coupling gel (hypromellose 0.3%, carbomer 980 0.2%, Ciba Vision Ophthalmics, Australia) were used between the objective lens and Tomocap contact cap.
Five best-focused images overlapping by less than 20% were selected for each location and one image from the inferior whorl (due to its small area).
Bright/ hyperreflective cells at least 10 µm in size, with a linear/curvilinear cell body, with or without dendrites (both short and long), located at the subbasal corneal epithelium and distributed among nerve fibres that do/ do not cross them or conjunctival epithelium were considered DC [21]. Density was counted manually by two experienced observers (ZT, AA) masked to group assignment and image location. Mean DC number in 5 images (or in the single inferior whorl image) was reported as cells/mm2. The mean value of two investigators was used.
Cell body size was graded as small (10–25 µm), medium (26–40 µm), or large (>40 µm), based on the largest cell body size observed, by a single observer (ZT) [21]. The presence of any dendrites, the presence of long dendrites, and the presence of thick dendrites was also recorded. The presence of clusters of DC at the conjunctiva was also noted. Images devoid of DC were excluded.
Inter-observer repeatability of DC density was examined. Two experienced observers (ZT, AA) independently assessed DC density in images from 66 participants.
Air quality assessment
Data were collected between July 2019 and January 2020. From October 2019 Sydney experienced wildfires which emitted extreme levels of smoke. To examine whether these confounded measurements air quality indices were accessed, including Air Quality 24-hour Index (AQI), concentration of PM10, PM 2.5, and nitrogen dioxide (NO2), from the New South Wales State Government Department of Planning, Industry, and Environment (https://www.dpie.nsw.gov.au/air-quality/air-quality-data-services/data-download-facility) (Supplementary Table 1).
Statistical analysis
SPSS software (version 26; SPSS Inc.) was used. Differences in symptoms and signs between the allergic and control groups were assessed using the Independent Sample t-test or Mann–Whitney U test.
Where DC density was not normally distributed, values were log-transformed adding 0.2 to values of zero. A linear mixed model with a random effect for individuals was used to examine differences in (log) DC density between groups and between locations. The fixed effect and the interaction between group and location were included. The interactions of group with age, sex or air quality were included in separate models to check the effect of these variables on between-group differences in DC density. Pairwise comparisons between groups and locations were obtained. P-values for multiple comparisons between locations were adjusted by Holm’s step-down Bonferroni method.
Mann–Whitney U test (for cell body size) and Fisher’s Exact Test (for presence of dendrites) were used to assess differences in DC morphology between groups. Friedman, and Wilcoxon Singed Ranked Test (for cell body size), Cochran’s Q test (for presence of dendrites) were used to assess differences in DC morphology across locations and the p-values for pairwise comparison between locations were adjusted by Holm’s step-down Bonferroni method.
The generalised linear mixed model for ordinal and binary outcomes that we planned on using for morphology did not converge. Therefore, smaller models within each location were estimated to assess the effect of age, sex, and air quality on DC morphology, by examining the interaction of these factors with participant group.
Associations between DC and symptoms and signs were initially assessed using a univariate Spearman’s correlation coefficient (for DC density and cell body size) and a Mann–Whitney U test (for presence of dendrites). Symptoms and signs that were significant at p < 0.01 (adjusted for multiple comparisons) were added to a multivariate generalised estimating equation (linear, ordinal, and binary logistic). A backward elimination modelling approach was used, and the final model included only those signs and symptoms that were significantly associated with DC density and morphology at p < 0.05.
Inter-observer agreement for DC density was examined using Bland and Altman plots and the coefficient of repeatability (CoR).
P < 0.05 was considered statistically significant.
Results
Sixty-six participants (range 19.0–65.0 years) completed the study: 33 in the allergic group (mean age 39.6 ± 14.5 years, 51% female) and 33 controls (mean age 33.5 ± 7.9 years, 60% female). There was no significant difference between groups in age (p = 0.2) or sex (p = 0.6). All allergic participants tested positive to at least one dust mite or pollen allergen, 55% were positive to both dust mites and pollen, 27% were positive to only dust mites and 6% were positive to pollen only (Supplementary Table 2).
Symptoms and signs
Ocular allergy symptoms, signs, and tear film characteristics are summarised in Table 1.
Allergic participants reported more symptoms of dryness, itchiness, stinging, watering, redness, and a need to rub eyes (p ≤ 0.03) but not burning. The AUAQ allergy symptom score was higher in allergic participants (p < 0.001), as were DEQ-5 scores (p = 0.01). OSDI scores were not different. Limbal, bulbar, and palpebral redness, bulbar conjunctival chemosis, palpebral follicles, and conjunctival staining were higher (p ≤ 0.002), and tear break-up time was lower (p = 0.02) in allergic participants. Epithelial disorders, staining, and bulbar conjunctival papillae were graded zero in all participants.
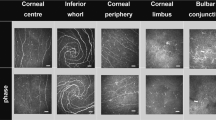
Topographical distribution of DC density
Representative IVCM images are shown in Fig. 1. The fixed effect of group was significant (F = 16.01, p < 0.001) showing that participant group has an impact on DC density. Simple main effects showed DC density was higher in allergic participants at all locations except at the inferior whorl (Fig. 2, Supplementary Table 3). The interactions between group and age (p = 0.35), sex (p = 0.15) or air quality (p ≥ 0.50) were not significant (Supplementary Table 4). Mean values for air quality indices are shown in Supplementary Table 1.
There was an effect of location on DC density (F = 91.92, p < 0.001). As the interaction between allergy group and location was not significant (F = 1.72, p = 0.15), the differences in DC density between locations were analysed together. The limbus had the highest DC density (p < 0.001), followed by the corneal periphery (p ≤ 0.01). The bulbar conjunctiva had the lowest DC density (p < 0.001) (Fig. 2 and Supplementary Table 3). There was no difference in DC density between the corneal centre and inferior whorl (p = 0.51).
DC morphology
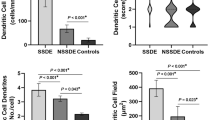
DC at the corneal centre had larger cell bodies (p = 0.02), and a higher proportion of DC with dendrites (p = 0.02) and with long dendrites (p = 0.003) in allergic participants (Fig. 3a, b). Although the proportion of DC with dendrites and with long dendrites appeared numerically greater for allergic participants at other locations, these differences were not significant (p ≥ 0.15). DC cell body size was not different between allergic and non-allergic participants at other locations (p ≥ 0.10). The presence of thick dendrites was not different between groups (p ≥ 0.20). The interactions between group and age (p ≥ 0.06), sex (p ≥ 0.06) or air quality (p ≥ 0.08) were not significant (Supplementary Table 4).
Dendritic cell (DC) body size (a) and dendrite appearance (presence of dendrites, presence of long and thick dendrites) (b) at corneal and conjunctival locations in allergy (n = 33) and control group (n = 33). Dendritic cell bodies were significantly larger at the corneal centre of allergic participants compared to the control group. Images devoid of DC were excluded thus data are presented for participants with dendritic cells at each location.
Conjunctival DC were present in 21 (64%) participants with allergy and 16 (48%) controls. In 38% of allergic participants DC gathered in a cluster and displayed a wire-netting pattern. This was not evident in controls.
In both groups there was a difference between locations in cell body size (p ≤ 0.01), presence of dendrites (p ≤ 0.001) and presence of long dendrites (p ≤ 0.002). Most conjunctival DC were of the highest-grade cell body size which was significantly larger than at all corneal locations (p ≤ 0.001) in the allergy group. DC at the corneal periphery and limbus had larger cell body size compared to the corneal centre in controls (p ≤ 0.04) and compared to the inferior whorl in the allergy group (p ≤ 0.04) (Fig. 3a).
In both groups, most DC at the corneal periphery, limbus, and bulbar conjunctiva presented with dendrites compared to DC at the corneal centre and inferior whorl (p ≤ 0.04).
Both groups had a higher proportion of long dendrites at the corneal periphery, limbus, and bulbar conjunctiva compared to the inferior whorl (p ≤ 0.04). More cells with long dendrites were observed at the bulbar conjunctiva compared to the corneal centre (p ≤ 0.03). Thick dendrites were rarely observed at the corneal periphery and limbus (Fig. 3b).
Associations between DC (density, morphology) and symptoms and signs
Eight weak correlations were observed at the univariate level (Supplementary Table 5). No associations were evident in the multivariate model. Among these, redness was the final covariate in the modelling steps with a p-value of 0.17.
No morphology parameter correlated with symptoms and signs (p ≤ 0.02, univariate level) (Supplementary Table 5).
Inter-observer repeatability of DC density
Figure 4 plots the differences in DC density between two observers against the mean of two measurements. The CoR of ±12.0 cells/mm2 at the corneal centre and the limits of agreement indicate that 95% of the differences between two observers can be expected to lie between +11.9 and −12.1 cells/mm2. The CoR was similar for the corneal periphery at ±14.3 cells/mm2, with relatively higher CoR values of ±29.1 cells/mm2 and ±28 cells/mm2 at the inferior whorl and limbus. The CoR was ±19.1 cells/mm2 for the conjunctiva. There was no bias between density measurements of two observers at corneal centre, periphery, limbus, or conjunctiva (p ≥ 0.3). The measurements of observer 1 were higher than observer 2 at the inferior whorl (p < 0.001) (Supplementary Table 6).
Discussion
DC density, morphology, and distribution were assessed in vivo, as a marker of allergic conjunctivitis. Corneal and conjunctival DC density increased during allergic conjunctivitis. DC at the corneal centre had larger cell bodies, and a higher proportion of DC had dendrites and long dendrites.
DC density was higher with allergic conjunctivitis compared to controls, however the difference was not as high as that previously reported between VKC and controls [10,11,12]. This is the first comprehensive report of DC density at different locations including bulbar conjunctiva in allergic conjunctivitis. In an IVCM study of patients with VKC, there was a higher DC density at the corneal centre (137.4 ± 27.0 cells/mm2) and limbus (148.8 ± 26.0 cells/mm2) compared to controls (24.1 ± 10.9 cells/mm2 and 98.2 ± 22.6 cells/mm2 respectively) [12]. A higher DC density has also been reported at the peripheral cornea (VKC; 403.3 ± 106.5; normal:83.3 ± 19.0 cells/mm2), and at the bulbar (VKC:244.0 ± 59.7; normal:18.0 ± 5.1 cells/mm2) and tarsal conjunctiva (VKC:232.2 ± 145.2; normal:0 cells/mm2) of patients with VKC compared to controls [10, 11]. These findings support a role for DC in the pathogenesis of ocular allergy and raise the possibility that changes in DC density and morphology could serve as biomarkers for allergic eye disease, its type and severity. Investigations that would include comparisons between all forms of allergic eye disease in a single study are warranted to confirm this.
DC morphology was altered at the corneal centre of allergic participants where cell bodies were larger, and a greater proportion of DC had dendrites and long dendrites. The study was not sufficiently powered to confirm similar morphological differences observed at other locations. A study on mice [18], and human IVCM studies found similar changes in DC morphology characterised by increased DC size or DC with more processes at the corneal centre of inflamed eyes but not at the corneal periphery [30, 31].
In 38% of allergic participants, a wire-netting pattern in DC morphology at the bulbar conjunctiva was observed. A similar finding was reported in VKC patients and this suggests conjunctival inflammation is present [11].
Morphological changes to DC have previously been reported during inflammation, including an increase in DC size, cell body size, length, thickness and number of dendrites, field area and cell area of dendrites [18, 19, 32,33,34,35]. In this study, changes in DC morphology characterised by a higher proportion of DC with dendrites and with long dendrites were observed at corneal locations of allergic participants, although significant changes were found only at the corneal centre. We previously showed a higher grade of DC morphology and DC with longer dendrites at the corneal centre of participants with systemic allergy (skin prick test positive) [20] probably related to their activation status and immune response. Activation of DC in vivo can only be determined based on their morphology as information about activation markers cannot be obtained in live human studies. However, evidence from immunohistochemistry studies supports use of DC morphological alteration as a hallmark of their activation [36].
Allergic immunity is dependent on the T cell response (Th2 cells, in particular) and T cell activation requires the interaction of T cell receptor with the major histocompatibility complex (MHC) class II on DC [37, 38]. An increased density of CD11c+ MHCII+ DC were observed in the nasal epithelium of allergic rhinitis patients sensitised to house dust mite [37]. Lung DC also play an essential role in the activation of Th2 cells and the development of allergic asthma [39]. A mice model of allergic conjunctivitis revealed the important role of ocular surface DC in triggering allergic immune responses and the therapeutic benefit of DC inhibition [40]. This study aligns with these models by confirming higher DC density and altered morphology in allergic conjunctivitis.
A gradient density from high DC density at the limbus to low density at the corneal centre and inferior whorl was observed. This aligns with previously reported in vivo findings in normal and inflamed corneas and ex vivo studies [11, 12, 22, 36, 41,42,43,44]. DC have been observed to move from the limbus to the corneal centre in porcine eyes in response to contact lens wear, and in mice following bone marrow cell transplantation [16, 17]. The bulbar conjunctiva had the lowest DC density compared to corneal locations in both groups. This aligns with previous reports [11, 22, 42] and animal work [45].
The distribution of DC morphology was different between locations for cell body size, presence of dendrites and presence of long dendrites with larger cell bodies and more cells with long dendrites at the corneal periphery, limbus, and bulbar conjunctiva compared to the corneal centre and inferior whorl in both groups. This distribution is suggestive of a higher antigen-capture capacity at the corneal periphery, limbus, and bulbar conjunctiva [21, 46]. MHC class II-negative DC were predominantly found in the corneal centre and MHC class II-positive DC at the corneal periphery and limbus in mice [47]. DC with thick dendrites show higher migratory capacity [21, 41, 48]. In this study, DC with thick dendrites were observed at the corneal periphery and limbus of both groups. Due to proximity of corneal limbus to the limbal vessels, DC can migrate toward drainage lymph nodes. In the mice model plasmacytoid DC patrolled the intravascular space [45].
In this study, allergic participants had mild/moderate symptoms and signs, but higher than controls. Participants with seasonal and perennial allergic conjunctivitis were enroled, both of which cause mild symptoms and signs (commonly itching and redness), which are not sight-threatening yet can significantly interfere in daily life and detrimentally affect quality of life [49]. Weak associations (univariate level) were reported between higher DC density and many elevated symptoms and signs in the allergy group but these were not retained at the multivariate level. There may be a relationship between DC and symptoms and signs of allergy, even in this group with allergic conjunctivitis which could be explored. Further work is required to establish whether certain DC characteristics can be considered as a biomarker of ocular allergic disease.
Inter-observer repeatability for DC density was good at the corneal centre, periphery, and bulbar conjunctiva and moderate for the corneal limbus and inferior whorl. We have reported a lower intra-observer repeatability coefficient for DC density at the limbus compared to the corneal centre and bulbar conjunctiva [22]. This study supports the reliability and reproducibility of IVCM assessment of DC. Strong inter-observer agreement was previously reported for central DC density in healthy participants and those with multiple sclerosis (intra-class correlation coefficient 0.99) [50]. Correlation coefficients measure the strength of association and not the agreement between measurements. This study’s CoR is an appropriate indicator of agreement providing insights into the inherent systematic errors of measurements of DC density obtained with IVCM.
This study used IVCM to assess DC density and morphology. While IVCM allows imaging of living tissues with high resolution and contrast, exact cell phenotype cannot be confirmed. Immunohistochemistry could facilitate validation of these IVCM results. The two-dimensional nature and small field of view of IVCM images continue to limit its application.
These results highlight the potential role of corneal and conjunctival DC in ocular allergy. Augmenting knowledge of DC function in ocular allergic inflammation may help develop new therapeutic approaches.
Summary
What was known before
-
Dendritic cells are the initiators of the allergic immune response.
-
Dendritic cell density is increased in vernal keratoconjunctivitis.
-
Dendritic cell density and morphology in the more common and often milder forms of allergic conjunctivitis have not been investigated.
What this study adds
-
This is the first study to assess the density, morphology, and distribution of corneal and conjunctival dendritic cells as a marker of allergic eye disease in allergic conjunctivitis using in vivo confocal microscopy Higher DC density was established at the cornea and conjunctiva in subjects with allergic conjunctivitis compared with unaffected controls.
-
There was increased antigen-capture capacity of corneal dendritic cells, evidenced by larger cell bodies and a higher proportion of cells with long dendrites which was established using a novel repeatable morphology grading system.
Data availability
The datasets generated during and/or analysed during the current study are available in the Mendeley Data repository, https://data.mendeley.com/drafts/ktmb6w7yb8.
Change history
22 March 2023
Missing Open Access funding information has been added in the Funding Note.
References
Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009;64:123–48.
Leonardi A, Castegnaro A, Valerio ALG, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15:482–8.
De Smedt S, Nkurikiye J, Fonteyne Y, Hogewoning A, Van Esbroeck M, De, et al. Vernal keratoconjunctivitis in school children in Rwanda and its association with socio-economic status: a population-based survey. Am J Trop Med Hyg. 2011;85:711–7.
Smith AF, Pitt AD, Rodruiguez AE, Alio JL, Marti N, Teus M, et al. The economic and quality of life impact of seasonal allergic conjunctivitis in a Spanish setting. Ophthalmic Epidemiol. 2005;12:233–42.
Saban DR, Calder V, Kuo C-H, Reyes NJ, Dartt DA, Ono SJ, et al. New twists to an old story: novel concepts in the pathogenesis of allergic eye disease. Curr Eye Res. 2013;38:317–30.
Manzouri B, Flynn T, Ohbayashi M, Ono SJ. The dendritic cell in allergic conjunctivitis. Ocul Surf. 2008;6:70–8.
von Bubnoff D, Koch S, Bieber T. Dendritic cells and atopic eczema/dermatitis syndrome. Curr Opin Allergy Clin Immunol. 2003;3:353–8.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52.
Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121:1132–40.
Le Q, Hong J, Zhu W, Sun X, Xu J. In vivo laser scanning confocal microscopy of vernal keratoconjunctivitis. Clin Exp Ophthalmol. 2011;39:53–60.
Liu M, Gao H, Wang T, Wang S, Li S, Shi W. An essential role for dendritic cells in vernal keratoconjunctivitis: analysis by laser scanning confocal microscopy. Clin Exp Allergy. 2014;44:362–70.
Mastropasqua L, Nubile M, Lanzini M, Carpineto P, Ciancaglini M, Pannellini T, et al. Epithelial dendritic cell distribution in normal and inflamed human cornea: in vivo confocal microscopy study. Am J Ophthalmol. 2006;142:736–44.
Kubaisi B, Samra K, Syeda S, Schmidt A, Foster S. Ocular allergy: an updated review. J Allergy Immunol. 2017;1:002.
Fukuda K, Nishida T. Ocular allergic inflammation: interaction between the cornea and conjunctiva. Cornea 2010;29:S62–7.
Leonardi A. Allergy and allergic mediators in tears. Exp Eye Res. 2013;117:106–17.
Nakamura T, Ishikawa F, Sonoda K-H, Hisatomi T, Qiao H, Yamada J, et al. Characterization and distribution of bone marrow–derived cells in mouse cornea. Investig Ophthalmol Vis Sci. 2005;46:497–503.
Sankaridurg PR, Rao GN, Rao HN, Sweeney DF, Holden BA. ATPase-positive dendritic cells in the limbal and corneal epithelium of guinea pigs after extended wear of hydrogel lenses. Cornea. 2000;19:374–7.
Jiao H, Naranjo Golborne C, Dando SJ, McMenamin PG, Downie LE, Chinnery HR. Topographical and morphological differences of corneal dendritic cells during steady state and inflammation. Ocul Immunol Inflamm. 2020;28:898–907.
Chinnery HR, Zhang XY, Wu CY, Downie LE. Corneal immune cell morphometry as an indicator of local and systemic pathology: a review. Clin Exp Ophthalmol. 2021;49:729–40.
Tajbakhsh Z, Jalbert I, Kolanu S, Stapleton F, Golebiowski B. Density and morphology of corneal epithelial dendritic cells are different in allergy. Curr Eye Res. 2019;45:675–9.
Chao C, Tajbakhsh Z, Stapleton F, Mobeen R, Madigan MC, Jalbert I, et al. Corneal epithelial dendritic cells, tear neuropeptides and corneal nerves continue to be affected more than 12 months after LASIK. Acta ophthalmologica. https://doi.org/10.1111/aos.15270. 2022.
Tajbakhsh Z, Jalbert I, Stapleton F, Briggs N, Golebiowski B Diurnal changes and topographical distribution in ocular surface epithelial dendritic cells in humans, and repeatability of density and morphology assessment. Ophthalmic Physiol Opt. https://doi.org/10.1111/opo.13087. 2022.
Nelson H. Diagnostic procedures in allergy. I: allergy skin testing. Ann Allergy. 1983;51:411–7.
Wolffsohn JS, Naroo SA, Gupta N, Emberlin J. Prevalence and impact of ocular allergy in the population attending UK optometric practice. Cont Lens Anterior Eye. 2011;34:133–8.
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–21.
Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33:55–60.
Terry RL, Schnider CM, Holden BA, Cornish R, Grant T, Sweeney D, et al. CCLRU standards for success of daily and extended wear contact lenses. Optom Vis Sci. 1993;70:234–43.
Takamura E, Uchio E, Ebihara N, Ohno S, Ohashi Y, Okamoto S, et al. Japanese guideline for allergic conjunctival diseases. Allergol Int. 2011;60:191–203.
Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50.
Lin H, Li W, Dong N, Chen W, Liu J, Chen L, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. 2010;51:122–8.
Wu L-Q, Cheng J-W, Cai J-P, Le Q-H, Ma X-Y, Gao L-D, et al. Observation of corneal langerhans cells by in vivo confocal microscopy in thyroid-associated ophthalmopathy. Curr Eye Res. 2016;41:927–32.
Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Jamali A, Hamrah P. Correlation of corneal immune cell changes with clinical severity in dry eye disease: an in vivo confocal microscopy study. Ocul Surf. 2021;19:183–9.
Lee EJ, Rosenbaum JT, Planck SR. Epifluorescence intravital microscopy of murine corneal dendritic cells. Invest Ophthalmol Vis Sci. 2010;51:2101–8.
Lagali NS, Badian RA, Liu X, Feldreich TR, Ärnlöv J, Utheim TP, et al. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci Rep. 2018;8:14248.
Levine H, Hwang J, Dermer H, Mehra D, Feuer W, Galor A. Relationships between activated dendritic cells and dry eye symptoms and signs. Ocul Surf. 2021;21:186–92.
Mayer WJ, Mackert MJ, Kranebitter N, Messmer EM, Gruterich M, Kampik A, et al. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr Eye Res. 2012;37:1012–8.
KleinJan A, Willart M, van Rijt LS, Braunstahl G-J, Leman K, Jung S, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118:1117–25.
Humeniuk P, Dubiela P, Hoffmann-Sommergruber K. Dendritic cells and their role in allergy: uptake, proteolytic processing and presentation of allergens. Int J Mol Sci. 2017;18:1491.
van Rijt LS, Jung S, KleinJan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–91.
Schlereth S, Lee HS, Khandelwal P, Saban DR. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am J Pathol. 2012;180:2351–60.
Jamali A, Seyed-Razavi Y, Chao C, Ortiz G, Kenyon B, Blanco T, et al. Intravital multiphoton microscopy of the ocular surface: alterations in conventional dendritic cell morphology and kinetics in dry eye disease. Front Immunol. 2020;11:742.
Mobeen R, Stapleton F, Chao C, Madigan MC, Briggs N, Golebiowski B. Corneal epithelial dendritic cell density in the healthy human cornea: a meta-analysis of in-vivo confocal microscopy data. Ocul Surf. 2019;17:753–62.
Rong B, Yan X-M. In vivo study of normal human limbal and central corneas using laser confocal microscope. Zhonghua Yan Ke Za Zhi. 2006;42:17–21.
Wang Y, Zhao F, Zhu W, Xu J, Zheng T, Sun X. In vivo confocal microscopic evaluation of morphologic changes and dendritic cell distribution in pterygium. Am J Ophthalmol. 2010;150:650–5.e1
Jamali A, Harris DL, Blanco T, Lopez MJ, Hamrah P. Resident plasmacytoid dendritic cells patrol vessels in the naïve limbus and conjunctiva. Ocul Surf. 2020;18:277–85.
Ward BR, Jester JV, Nishibu A, Vishwanath M, Shalhevet D, Kumamoto T, et al. Local thermal injury elicits immediate dynamic behavioural responses by corneal Langerhans cells. Immunology. 2007;120:556–72.
Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43:639–46.
Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N, et al. Dynamics and function of langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating langerhans cells. Immunity 2005;22:643–54.
Sánchez-Hernández M, Montero J, Rondon C, Benitez del Castillo J, Velázquez E, Herreras J, et al. Consensus document on allergic conjunctivitis (DECA). J Investig Allergol Clin Immunol. 2015;25:94–106.
Kheirkhah A, Muller R, Mikolajczak J, Ren A, Kadas EM, Zimmermann H, et al. Comparison of standard versus wide-field composite images of the corneal subbasal layer by In vivo confocal microscopy. Investig Ophthalmol Vis Sci. 2015;56:5801–7.
Acknowledgements
The authors acknowledge the Eye Research Group at the School of Optometry and Vision Science for supporting this research through the provision of clinical facilities. The authors are grateful to Dr. Thomas John Naduvilath for assistance with some of the statistical analyses.
Funding
This research did not receive specific funding from agencies in the public, commercial, or not-for-profit sectors. The first author received a fee remission scholarship from UNSW Sydney and the Australian Government Research Training Program Scholarship. This study was also supported by the University of New South Wales (UNSW) and the Faculty of Science Research Infrastructure Scheme. The funding sources have no involvement in the study design, conduct of the research, collection, analysis and interpretation of data, writing of the report, preparation of the article or in the decision to submit the article for publication. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
ZT contributed substantially to the conception and design of this work, she was responsible for; data acquisition, data analysis, and interpretation, drafting the manuscript, and revising it critically for important and intellectual content. IJ, FS, and BG contributed substantially to the conception and design of this work, they were responsible for; the interpretation of data for the work and revising the manuscript critically for important and intellectual content. AA contributed to data analysis, and critical review of the manuscript. BG and NB contributed substantially to data analysis and interpretation of the results. PG and BA facilitated data acquisition and reviewed the manuscript critically. ZT, BG, FS, AA, PG, BA, NB, and IJ approved the final version of this manuscript submitted to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tajbakhsh, Z., Golebiowski, B., Stapleton, F. et al. Increased dendritic cell density and altered morphology in allergic conjunctivitis. Eye 37, 2896–2904 (2023). https://doi.org/10.1038/s41433-023-02426-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02426-x