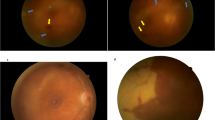
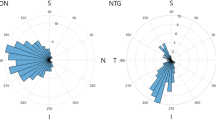
Abstract
Background/Aims
To identify characteristics that can distinguish AAION from NAAION in emergency practice.
Methods
This is a multicentre retrospective case-control study. Ninety-four patients with AAION were compared to ninety-four consecutive patients with NAAION. We compared the clinical, biological, and ophthalmological characteristics at baseline of patients with AAION and those with NAAION.
Results
Patients with AAION were older and more likely to have arterial hypertension. Cephalic symptoms and acute-phase reactants were more frequent in AAION. Profound vision loss and bilateral involvement were more frequent in AAION at baseline. Central retinal and cilioretinal artery occlusions was only observed in AAION, and delayed choroidal perfusion was more frequently observed in AAION than in NAAION. Using logistic regression, an age >70 years (OR = 3.4, IC95% = 0.8–16.1, p = 0.105), absence of splinter haemorrhage (OR = 4.9, IC95% = 1.4–20.5, p = 0.019), delayed choroidal perfusion (OR = 7.2, IC95% = 2.0–28.0, p = 0.003), CRP > 7 mg/L (OR = 43.6, IC95% = 11.6–229.1, p < 0.001) and platelets >400 × G/L (OR = 27.5, IC95% = 4.6–270.9, p = 0.001) were independently associated with a diagnosis of AAION. An easy-to-use score based on these variables accurately distinguished AAION from NAAION with a sensitivity of 93.3% and specificity of 92.4%.
Conclusion
In patients presenting with AION, a set of ophthalmological and laboratory criteria can efficiently discriminate patients with AAION and NAAION and can identify which patients would benefit from high-dose glucocorticoids. External validation of our results is required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Authors confirm that the data supporting the findings of this study are available within the article.
References
Hayreh SS Ischemic optic neuropathies. Springer, Heidelberg 2011.
Hayreh SS, Joos KM, Podhajsky PA, Long CR. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1994;118:766–80.
Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371:50–57.
Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30.
Atkins EJ, Bruce BB, Newman NJ, Biousse V. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol. 2010;55:47–63.
Hayreh SS, Podhajsky PA, Zimmerman B. Occult giant cell arteritis: ocular manifestations. Am J Ophthalmol. 1998;125:521–6.
Koçak N, Yeter V, Güngör I. Monocyte to high-density lipoprotein ratio in patients with arteritic and non-arteritic anterior ischaemic optic neuropathy. Neuroophthalmology. 2020;44:294–8.
Inanc M, Tekin K, Budakoglu O, Ilhan B, Aydemir O, Yilmazbas P. Could platelet indices and neutrophil to lymphocyte ratio be new biomarkers for differentiation of arteritic anterior ischemic neuropathy from non-arteritic type? Neuroophthalmology. 2018;42:287–94.
Liozon E, Herrmann F, Ly K, Robert PY, Loustaud V, Soria P, et al. Risk factors for permanent visual loss in giant cell (temporal) arteritis: a prospective study of 174 patients. Am J Med. 2001;111:211–7.
Costello F, Zimmerman MB, Podhajsky PA, Hayreh SS. Role of thrombocytosis in diagnosis of giant cell arteritis and differentiation of arteritic from non-arteritic anterior ischemic optic neuropathy. Eur J Ophthalmol. 2004;14:245–57.
Jonas JB, Gusek GC, Naumann GO. Anterior ischemic optic neuropathy: nonarteritic form in small and giant cell arteritis in normal sized optic discs. Int Ophthalmol. 1988;12:119–25.
Monteiro ML. Anterior ischemic optic neuropathy: a comparison of the optic disc area of patients with the arteritic and non-arteritic forms of the disease and that of normal controls. Arq Bras Oftalmol. 2006;69:805–10.
Danesh-Meyer HV, Boland MV, Savino PJ, Miller NR, Subramanian PS, Girkin CA, et al. Optic disc morphology in open-angle glaucoma compared with anterior ischemic optic neuropathies. Invest Ophthalmol Vis Sci. 2010;51:2003–10.
Balducci N, Morara M, Veronese C, Barboni P, Casadei NL, Savini G, et al. Optical coherence tomography angiography in acute arteritic and non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:2255–61.
Pellegrini M, Giannaccare G, Bernabei F, Moscardelli F, Schiavi C, Campos EC. Choroidal vascular changes in arteritic and nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2019;205:43–49.
Siatkowski RM, Gass JD, Glaser JS, Smith JL, Schatz NJ, Schiffman J. Fluorescein angiography in the diagnosis of giant cell arteritis. Am J Ophthalmol. 1993;115:57–63.
Valmaggia C, Speiser P, Bischoff P, Niederberger H. Indocyanine green versus fluorescein angiography in the differential diagnosis of arteritic and nonarteritic anterior ischemic optic neuropathy. Retina 1999;19:131–4.
Jonas JB, Harder B. Central retinal artery and vein collapse pressure in giant cell arteritis versus nonarteritic anterior ischaemic optic neuropathy. Eye (Lond). 2008;22:556–8.
Huna-Baron R, Mizrachi IB, Glovinsky Y. Intraocular pressure is low in eyes with giant cell arteritis. J Neuroophthalmol. 2006;26:273–5.
Ghanchi FD, Williamson TH, Lim CS, Butt Z, Baxter GM, McKillop G, et al. Colour Doppler imaging in giant cell (temporal) arteritis: serial examination and comparison with non-arteritic anterior ischaemic optic neuropathy. Eye (Lond). 1996;10:459–64.
Remond P, Attyé A, Lecler A, Lamalle L, Boudiaf N, Aptel F, et al. The central bright spot sign: a potential new mr imaging sign for the early diagnosis of anterior ischemic optic neuropathy due to giant cell arteritis. Am J Neuroradiol. 2017;38:1411–5.
Danesh-Meyer HV, Savino PJ, Sergott RC. The prevalence of cupping in end-stage arteritic and nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2001;108:593–8.
Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–228.
de Boysson H, Dumont A, Liozon E, Lambert M, Boutemy J, Maigné G, et al. Giant-cell arteritis: Concordance study between aortic CT angiography and FDG-PET/CT in detection of large-vessel involvement. Eur J Nucl Med Mol Imaging. 2017;44:2274–9.
Rinagel M, Chatelus E, Jousse-Joulin S, Sibilia J, Gottenberg JE, Chasset F, et al. Diagnostic performance of temporal artery ultrasound for the diagnosis of giant cell arteritis: a systematic review and meta-analysis of the literature. Autoimmun Rev. 2019;18:56–61.
Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636–43.
Chan FLY, Lester S, Whittle SL, Hill CL. The utility of ESR, CRP and platelets in the diagnosis of GCA. BMC Rheumatol 2019;3:14 https://doi.org/10.1186/s41927-019-0061-z
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72.
Van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67.
Ing EB, Lahaie Luna G, Toren A, Ing R, Chen JJ, Arora N, et al. Multivariable prediction model for suspected giant cell arteritis: development and validation. Clin Ophthalmol. 2017;11:2031–42.
Ing E, Su W, Schonlau M, Torun N. Support Vector Machines and logistic regression to predict temporal artery biopsy outcomes. Can J Ophthalmol. 2019;54:116–8.
Ing EB, Ing R. The use of a nomogram to visually interpret a logistic regression prediction model for giant cell arteritis. Neuroophthalmology. 2018;42:284–6.
Ing EB, Miller NR, Nguyen A, Su W, Bursztyn LLCD, Poole M, et al. Neural network and logistic regression diagnostic prediction models for giant cell arteritis: development and validation. Clin Ophthalmol. 2019;13:421–30.
Czihal M, Tschaidse J, Bernau C, Lottspeich C, Köhler A, Dechant C, et al. Ocular ischaemic complications in giant cell arteritis: CHADS2-score predicts risk of permanent visual impairment. Clin Exp Rheumatol. 2019;37:61–64.
Hayreh SS. Anterior ischaemic optic neuropathy. differentiation of arteritic from non-arteritic type and its management. Eye. 1990;4:25–41.
Mack HG, O’Day J, Currie JN. Delayed choroidal perfusion in giant cell arteritis. J Clin Neuroophthalmol. 1991;11:221–7.
Bei L, Lee I, Lee MS, Van Stavern GP, McClelland CM. Acute vision loss and choroidal filling delay in the absence of giant-cell arteritis. Clin Ophthalmol. 2016;10:1573–8.
Chen JJ, Leavitt JA, Fang C, Crowson CS, Matteson EL, Warrington KJ. evaluating the incidence of arteritic ischemic optic neuropathy and other causes of vision loss from giant cell arteritis. Ophthalmology. 2016;123:1999–2003.
Author information
Authors and Affiliations
Contributions
Conception and design of the work: SP, TS, BT Acquisition and interpretation of the data: SP, AD, RM, SD, AR, GG, DM, APB, KHL, EL, TS, BT. Drafting the manuscript: SP, BT. Revising the work critically: AD, RM, SD, AR, GG, DM, APB, KHL, EL, TS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parreau, S., Dentel, A., Mhenni, R. et al. Clinical, biological, and ophthalmological characteristics differentiating arteritic from non-arteritic anterior ischaemic optic neuropathy. Eye 37, 2095–2100 (2023). https://doi.org/10.1038/s41433-022-02295-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02295-w