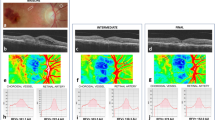
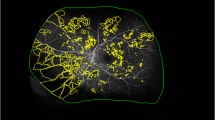
Abstract
Objectives
To evaluate the macular vascular flow in eyes with idiopathic macular pucker (EyeiMP), pre and post pars plana vitrectomy with epiretinal and limiting membranes peeling, and to compare it with the vascular flow in the healthy fellow eyes (Eyefellow), taken as physiological reference value.
Methods
40 eyes of 40 patients were recruited. Best-corrected visual acuity (BCVA) was evaluated. Spectral domain optical coherence tomography (SD-OCT) and OCT-angiography parameters were central foveal thickness (CFT), choroidal thickness (CT), foveal avascular zone (FAZ) area, vessel area density (VAD), vessel length fraction (VLF), vessel density index (VDI) of superficial capillary plexus (SCP) and deep vascular complex (DVC), choriocapillaris (CC) flow. Absolute and relative difference calculation was applied to evaluate macular vascular flow in EyeiMP adjusted for physiological changes detected in Eyefellow. Follow-up: 6 months.
Results
BCVA improved (p = 0.003) in all cases following surgery. CFT reduced postoperatively (p = 0.0138). FAZ area was smaller in EyeiMP than Eyefellow (p = 0.0071) preoperatively and postoperatively it shrank further (p = 0.0027). After surgery, inverse correlation between FAZ area and BCVA was detected (r-0.683). VAD of SCP was pre- and post-operatively higher in EyeiMP than Eyefellow (baseline p = 0.0344, 6th month p = 0.0466). Relative difference of VDI of SCP (p = 0.0096) and CC flow (p = 0.0013) at 6 months reduced. DVC flow changed significantly only in Eyefellow. CT increased post-operatively in both EyeiMP (p = 0.0345) and Eyefellow (p = 0.00423), but relative difference did not change.
Conclusions
Vascular flow indices monitoring demonstrated significant changes in both eyes: EyeiMP and Eyefellow. Relative difference of vascular flow provided objective estimate of changes due to iMP surgery taking into account physiological changes in Eyefellow.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data are available upon request and are with the first author.
References
Pournaras CJ, Emarah A, Petropoulos IK. Idiopathic macular epiretinal membrane surgery and ILM peeling: Anatomical and functional outcomes. Semin Ophthalmol. 2011;26:42–6. https://doi.org/10.3109/08820538.2010.544237.
Rahman R, Stephenson J. Early surgery for epiretinal membrane preserves more vision for patients. Eye. 2014;28:410–4. https://doi.org/10.1038/eye.2013.305.
Yoon YS, Woo JM, Woo JE, Min JK. Superficial foveal avascular zone area changes before and after idiopathic epiretinal membrane surgery. Int J Ophthalmol. 2018;11:1711–5. https://doi.org/10.18240/ijo.2018.10.21.
Kitagawa Y, Shimada H, Shinojima A, Nakasashizuka H. Foveal avascular zone area analysis using optical coherence tomography angiography before and after idiopathic epiretinal membrane surgery. Retina. 2019;39:339–46. https://doi.org/10.1097/IAE.0000000000001972.
Kumagai K, Furukawa M, Suetsugu T, Ogino N. Foveal avascular zone area after internal limiting membrane peeling for epiretinal membrane and macular hole compared with that of fellow eyes and healthy controls. Retina. 2018;38:1786–94. https://doi.org/10.1097/IAE.0000000000001778.
Okawa Y, Maruko I, Kawai M, Hasegawa T, Arakawa H, Lida T. Foveal structure and vasculature in eyes with idiopathic epiretinal membrane. PLoS ONE. 2019;14:1–8. https://doi.org/10.1371/journal.pone.0214881.
Romano MR, Cennamo G, Schiemer S, Rossi C, Sparnelli F, Cennamo G. Deep and superficial OCT angiography changes after macular peeling: idiopathic vs diabetic epiretinal membranes. Graefes Arch Clin Ophthalmol. 2017;255:681–9. https://doi.org/10.1007/s00417-016-3534-4.
Kim YJ, Kim S, Lee JY, Kim JG, Yoon YH. Macular capillary plexuses after epiretinal membrane surgery: an optical coherence tomography angiography study. Br J Ophthalmol. 2017;102:1086–91. https://doi.org/10.1136/bjophthalmol-2017-311188.
Kumagai K, Ogino N, Furukawa M, Ooya R, Horie E. Early centripetal displacements of capillaries in macular region caused by internal limiting membrane peeling. Clin Ophthalmol. 2018;12:755–63. https://doi.org/10.2147/OPTH.S158826.
Chen H, Chi W, Cai X, Deng Y, Jiang X, Wei Y, et al. Macular microvasculature features before and after vitrectomy in idiopathic macular epiretinal membrane: an OCT angiography analysis. Eye. 2019;33:619–28. https://doi.org/10.1038/s41433-018-0272-3.
Mastropasqua L, Borrelli E, Carpineto P, Toto L, Di Antoio L, Mattei PA, et al. Microvascular changes after vitrectomy with internal limiting membrane peeling: an optical coherence tomography angiography study. Int Ophthalmol. 2018;38:1465–72. https://doi.org/10.1007/s10792-017-0608-1.
Casini G, Lazzeri S. Analysis of choroidal thickness change after 25-gauge vitrectomy for idiopathic epiretinal membrane with or without phacoemulsification and intraocular lens implantation. Ophthalmologica. 2017;237:78–84. https://doi.org/10.1159/000452769.
Ahn SJ, Woo SJ, Park KH. Choroidal thickness change following vitrectomy in idiopathic epiretinal membrane and macular hole. Graefes Arch Clin Exp Ophthalmol. 2016;254:1059–67. https://doi.org/10.1007/s00417-015-3154-4.
Yu Y, Teng Y, Gao M, Liu X, Chen J, Liu W. Quantitative choriocapillaris perfusion before and after vitrectomy in idiopathic epiretinal membrane by optical coherence tomography angiography. Ophthalmic Surg, Lasers Imaging Retin. 2017;48:906–15. https://doi.org/10.3928/23258160-20171030-06.
Michalewska Z, Michalewski J, Ornafel-Sagan K, Navrocki J. Swept-source optical coherence tomography correlations between retina and choroid before and after vitrectomy for epiretinal membranes. Am J Ophthalmol. 2016;165:100–7. https://doi.org/10.1016/j.ajo.2016.02.003.
Michalewska Z, Michalewski J, Adelman R, Zawislak E, Navrocki J. Choroidal thickness measured with swept source optical coherence tomography before and after vitrectomy with internal limiting membrane peeling for idiopathic epiretinal membranes. Retina. 2015;35:487–91.
Gass JDM. Macular dysfunction caused by epiretinal membrane contraction. In: Stereoscopic atlas of macular diseases: diagnosis and treatment. 4th ed., vol. 2. St Louis, Mo: Mosbyy; 1997. p. 938–50.
Govetto A, Lalane RA III, Sarraf D, Figueroa MS, Hubschman JP. Insights into epiretiinal membranes: presence of ectopic inner foveal layers and a neww optical coherence tomography staging scheme. Am J Ophthalmol. 2017;175:99–113. https://doi.org/10.1016/j.ajo.2016.12.006.
Reif R, Qin J, An L, Zhi Z, Dziennis S, Wang R. Quantifying optical microangiography images obtained from a spectral domain optical coherence tomography system. Int J Biomed Imaging. 2012;2012:509783. https://doi.org/10.1155/2012/509783.
Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57:OCT362.70. https://doi.org/10.1167/iovs.15-18904.
Phansalkar N, More S, Sabale A, Joshi M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. 2011 International Conference on Communications and Signal Processing, 2011, p. 218–220. https://doi.org/10.1109/ICCSP.2011.5739305.
Spaide RF. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol. 2016;170:58–67. https://doi.org/10.1016/j.ajo.2016.07.023.
Zouache MA, Eames I, Klettner CA, Luthert PJ. Form, shape and function: segmented blood flow in the choriocapillaris. Sci Rep. 2016;6:35754. https://doi.org/10.1038/srep35754.
Aleksic M, Matoussevitch V, Heckenkamp J, Brunkwall J. Changes in internal carotid blood flow after CEA evaluated by transit-time flowmeter. Eur J Endoscopic Surg. 2006;31:14–7. https://doi.org/10.1016/j.ejvs.05.08.029.
Eckstein HH, Eichbaum M, KlemmK, Doerfler A, Ringleb P, Bruckner T, et al. Improvement of carotid blood flow after carotid endarterectomy—evaluation using intraoperative ultrasound flow measurement. Eur J Vasc Endovasc Surg. 2003;25:168–74. https://doi.org/10.1053/ejvs.2002.1820.
Author information
Authors and Affiliations
Contributions
RF—conceptualization, writing of the paper, supervision. LT, IG, and JYS—collection and analysis of the data, writing of the paper. BP—formal analysis, review and editing. GDS—writing of the paper, review. AM—data curation, supervision. All authors have read and agreed to the published version of the paper.
Corresponding author
Ethics declarations
Competing interests
RF, LT, IG, JYS, BP, and AM certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials. GDS received honoraria and consultancies fees outside the current work from the following companies: Allergan Abbvie, Apellis, Bayer, Boehringer Ingelheim, Heidelberg Engineering, Novartis.
Ethics approval and consent to participate
All research and measurements adhered to the tenets of the Declaration of Helsinki and written informed consent was obtained for each participant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frisina, R., De Salvo, G., Tozzi, L. et al. Effects of physiological fluctuations on the estimation of vascular flow in eyes with idiopathic macular pucker. Eye 37, 1470–1478 (2023). https://doi.org/10.1038/s41433-022-02158-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02158-4