Abstract
Objectives
To compare the visual outcome of patients treated for non-arthritic central retinal artery occlusion (CRAO) in a medical centre that uses hyperbaric oxygen therapy (HBOT) as part of the standard of care (SOC) to medical centres that does not.
Methods
The study included data from two tertiary medical centres. The medical records of all patients diagnosed with non-arthritic CRAO without a patent cilioretinal artery between January 2010 and December 2018 in two tertiary medical centres were reviewed.
Results
One hundred and twenty-one patients were treated by HBOT and 23 patients received only SOC. In the HBOT group, best-corrected visual acuity (BCVA) improved from 2.89 ± 0.98 logMAR at presentation to 2.15 ± 1.07 logMAR upon the end of HBOT (P < 0.001), while the SOC group had no significant improvement, from 3.04 ± 0.82 logMAR at presentation to 2.80 ± 1.50 logMAR (P = 0.24). With adjustment for age, gender, and the duration of symptoms, final BCVA in the HBOT group was significantly better compared to the control group (P = 0.023). Rates of patients achieving vision of 20/200 or better were similar between groups (17.4% vs. 19.8%, P = 0.523).
Conclusion
Utilizing HBOT as part of the SOC for CRAO improves the final visual outcome. HBOT is safe and can be implemented, if available, as part of SOC in all tertiary medical centres.
Similar content being viewed by others
Introduction
The central retinal artery is responsible for the blood supply to the inner two-thirds of the retina. Being a functional end artery, an occlusion or obstruction of this vessel leads to a sudden, painless visual loss. The incidence of central retinal artery occlusion (CRAO) is ~1–2 in 100,000 [1].
According to a consensus statement published by The American Heart Association [2] central nervous system infarction (stroke) is defined as ‘brain, spinal cord or retinal cell death attributable to ischaemia, based on neuro-pathological, neuroimaging and/or clinical evidence of permanent injury’. Moreover, acute cerebral infractions were found in 27–76.4% of CRAO patients with magnetic resonance imaging with diffusion-weighted imaging [3]. Therefore, CRAO is a stroke equivalent and represents an ophthalmologic and medical emergency.
A variety of measures have been used in an attempt to treat CRAO. These include lowering IOP using anterior chamber paracentesis, or pressure-lowering medication to promote dislodging of the embolus downstream [4]. Vasodilatation of the retinal vessels using sublingual isosorbide dinitrate and carbon dioxide or carbogen inhalation [5]. Recently intra-arterial fibrinolysis (LIF), surgical embolectomy or neodymium:yttrium–aluminium–garnet laser embolysis were described [6,7,8]. Convincing evidence demonstrating the efficacy of any particular intervention has yet to be found.
Hyperbaric oxygen therapy (HBOT) includes the inhalation of 100% oxygen at pressures exceeding 1 atmosphere absolute (ATA) used to enhance the amount of oxygen dissolved in the body tissues. During HBOT treatment, the arterial O2 tension typically exceeds 1700 mmHg and the dissolved oxygen in the blood can be increased from 0.3 to 6 vol% [9]. The proposed role for hyperbaric oxygen in CRAO is to increase the oxygen delivery to the ischaemic tissue until spontaneous or assisted reperfusion occurs.
Several case series in which HBOT has been used in CRAO have been reported [10,11,12,13,14,15,16]. Their authors suggested that hyperbaric oxygen treatment shows beneficial effects on visual acuity (VA) while entailing a relatively low-risk profile, with the strongest evidence when administrated within the first 8–12 h from the onset of the visual loss. Most of these reports were case series with relatively small sample size.
The purpose of our study is to report the visual outcomes of patients treated with hyperbaric oxygen for non-arteritic CRAO and compare their outcomes to patients treated by the standard of care (SOC).
Materials and methods
The study included data from two tertiary medical centres in Israel, Shamir Medical Center that has the Sagol Center for hyperbaric medicine and Soroka Medical Center that does not have a hyperbaric facility. The data were collected retrospectively from January 2010 to December 2018. Shamir Medical Center and Soroka Medical Center Institutional Review Boards approval was obtained for retrospective analysis of all cases used in this study, as well as waived patient consent.
The data collected retrospectively included age, sex, systemic risk factors, chronic medications, the time between the onset of symptoms to treatment, funduscopic findings, best-corrected visual acuity (BCVA) at presentation, at the end of HBOT or at discharge from the hospital and at the last recorded follow-up visit, intraocular pressure (IOP) at presentation and adverse events.
Patients were included if they were older than 18 years and had complete or subtotal non-arteritic CRAO with symptoms lasting for <24 h. Patients with a patent cilioretinal artery were excluded from the study as were those with arteritic CRAO. Further exclusion criteria were absence of documented BCVA, iatrogenic CRAO and branch retinal artery occlusion.
All patients underwent a complete ocular examination upon presentation including a Snellen BCVA by a certified ophthalmologist, applanation tonometry, pupillary response, slit-lamp exam and dilated fundus examination. A complete ocular examination was repeated at the end of HBOT or before discharge from the hospital and on each follow-up visit.
The diagnosis of CRAO was made clinically based on the classic presentation of acute, painless onset of visual loss, relative afferent pupillary defect and fundus examination showing opaque and oedematous retina in the posterior pole. Fluorescine angiography (Topcon 50EX camera; excitation wavelength between 465 and 490 nm, emission of 520–530 nm; OIS WinStation 5000TM software [Ophthalmic Imaging Systems, Sacramento, CA]) was used to confirm the diagnosis in cases that a cherry-red spot was not evident.
Hyperbaric oxygen protocol
The HBOT protocol included three sessions within 24 h (8 h apart), while the first session was at 2.4 ATA and the rest of the sessions at 2 ATA of 100% oxygen for 90-min sessions, with 5 min air breaks every 20 min. Snellen BCVA was recorded by a certified ophthalmologist after each treatment session. After three sessions, HBOT continued once daily until no further improvement in BCVA was observed in two consecutive treatments. All HBOT sessions were performed in a multiplace hyperbaric chamber (Starmed 2700, HAUX-Life Support-GmbH, Germany) the Sagol Center for hyperbaric medicine and research.
Additional treatments
All patients underwent a neurological assessment that included neuroimaging according to a certified neurologist decision.
All patients, in both medical centres, were treated with the SOC treatment that included: ocular massage, anterior chamber paracentesis, oral aspirin, oral acetazolamide or topical beta-blocker according to a certified ophthalmologist’s decision.
Statistical analysis
The baseline and outcome variables were compared, between the two groups, with the use of Student’s t test, the chi-square test, and the Mann–Whitney U test, as appropriate. Normal distribution was evaluated with Q–Q plot, Shapiro–Wilk test and Histogram chart with a normal distribution curve. For the multivariable analysis, we used logistic regression that was used with the likelihood ratio test to choose which confounders to retain in the model. Results were considered as significant if the P value was below 0.05.
All analyses were performed using IBM SPSS Statistics for Windows version 24.0 (IBM Corp., Armonk, NY, USA).
Results
One hundred and thirty-four patients were included in the study. From them, 121 patients were treated with HBOT in addition to the acceptable SOC (HBOT group) and 23 patients were treated only with the SOC treatment with no HBOT (control group). All cases included had a complete or subtotal non-arteritic CRAO without a patent cilioretinal artery. The baseline characteristics of both groups are summarized in Table 1. There was no significant difference between the groups as to any of the following parameters: systemic vascular risk factors, presenting BCVA, intraocular pressure at presentation, presence of a cherry-red spot or diabetic retinopathy. The HBOT group was significantly older than the control group (69 ± 12 vs. 60 ± 3 years, P = 0.002) and had a significantly shorter duration of symptoms (9.1 ± 5 vs. 19 ± 18 h, P = 0.003).
The HBOT group received a median of 4 hyperbaric oxygen treatments (range 2–8). The mean follow-up time was 12.9 ± 34 months for the treatment group and 51.5 ± 57 months for the control group.
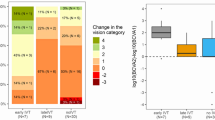
Figure 1 summarizes the VA outcomes of the HBOT and control groups. In the HBOT group, BCVA improved from 2.89 ± 0.98 logMAR at presentation to 2.15 ± 1.07 logMAR upon the end of hyperbaric oxygen treatment (P < 0.001). This improvement remained statistically significant upon the last follow-up (P < 0.001). In the control group, BCVA changed from 3.04 ± 0.82 logMAR at presentation to 2.80 ± 1.50 logMAR upon the last follow-up, this change was not found to be statistically significant (P = 0.24). With adjustment for age, gender and the duration of symptoms, final BCVA was significantly better in the HBOT group compared to the control group 2.27 ± 1.25 logMAR and 2.80 ± 1.50 logMAR respectively (P = 0.023).
Seventeen percent of patients in the control group and 20% of patients in the HBOT group had achieved BCVA of 20/200 or better (P = 0.523). The distribution of the final BCVA of the HBOT and control groups is illustrated in Fig. 2.
Comparison of best-corrected visual acuity (BCVA) distribution between patients with non-arteritic central retinal artery occlusion who received hyperbaric oxygen treatment (HBOT group) and no treatment (control group) at the last follow-up visit. Box = percentage of eye with the denoted visual acuity at the last follow-up visit.
Within the HBOT group, the mean time between the onset of symptoms and the first hyperbaric oxygen treatment was 9.1 ± 5 h. No correlation was found between the time to the first hyperbaric oxygen treatment and BCVA at the end of treatment (P = 0.32). The mean final BCVA of patients that underwent the first hyperbaric oxygen treatment within 6 h from the onset of symptoms was 2.44 ± 1.14 loMAR while the mean final BCVA of the patient who underwent the first treatment between 6 and 24 h was 2.18 ± 1.31 logMAR (P = 0.30).
No association was found between clinical characteristics at presentation and the final BCVA of the HBOT group. There was no significant correlation between the presence of a cherry-red spot at presentation or diabetic retinopathy with the final VA (P = 0.45, P = 0.23, respectively).
Three patients (0.02%) had stopped hyperbaric oxygen treatment prematurely. Two patients due to ear barotrauma and one due to an episode of seizures and epistaxis during treatment.
Discussion
Spontaneous improvement of VA has been reported in up to 37% of patients with CRAO [17]. In the EAGLE study [6], a prospective randomized multicentre clinical trial to compare treatment outcome after conservative standard treatment and LIF for acute non-arteritic CRAO, the BCVA of the conservative standard treatment group was improved by 0.443 logMAR within 1 month. Thus, presenting improvement in VA might not indicate the potency of a treatment for these patients and might merely reflect the natural history of the disease. For this reason, in this study, the visual outcome of CRAO was compared between two tertiary medical centres: one that uses HBOT as part of its SOC and the other that does not.
Beiran et al. [13] had retrospectively compared the outcomes of 30 patients treated with HBOT with the outcomes of 35 matched retinal artery occlusion patients form a different medical centre that were not treated with HBOT. They found a significantly better final BCVA in the treatment group (0.29 vs. 0.13 logMAR). However, this series included all retinal artery occlusion categories including branch occlusion, patent cilioretinal artery and arteritic CRAO, whereas our report includes only complete or subtotal non-arthritic CRAO without a patent cilioretinal artery.
We found in our series a significant improvement of VA (0.53 logMAR) in patients treated with HBOT for CRAO. This improvement is consistent with previous studies. Menzel-Severing et al. [10] found a 0.2-logMAR improvement in 51 patients treated with a combination of HBOT and haemodilution. Hadanny et al. [11] found a 0.526-logMAR improvement in 120 patients treated for CRAO.
A mean VA improvement between presentation and last follow-up visit of 0.15 logMAR was not found to be statistically significant in the control group (that was not treated with HBOT). With adjustment for age, gender and the duration of symptoms, final BCVA of the HBOT group was significantly better compared to the control group (2.27 ± 1.25 vs. 2.80 ± 1.50, P = 0.023). These results indicate that VA improvement found in patients treated with HBOT is attributed to more than just the natural history of the disease.
The time window for an effective hyperbaric oxygen treatment is extremely important in light of the guidelines published by the American Academy of Ophthalmology in 2018 [3], which recommended that every CRAO patient should undergo neuroimaging within 24 h of symptoms onset. Previous reports have suggested that the time of the first HBOT and the lack of the presence of a cherry-red spot are indicators for a better visual outcome with HBOT [11]. Hadanny et al. reported a linear correlation between the presence of a cherry-red spot and final BCVA, in their series, the presence of a cherry-red spot decreased the gain in logMAR by 0.787. Also, in that series time delay from symptoms to treatment had a significant effect on the final BCVA (0.03 logMAR for each hour of delay). This correlation is supported by a report by Hayreh et al. [18] who studied the effects of central retina artery clamping on rhesus monkey’s retinal survival time and found massive irreversible damage after 4 h of clumping.
In our series, we did not find such a correlation between the time of the first hyperbaric oxygen treatment or the presence of a cherry-red spot and final visual outcome. This might be due to the fact that all patients commenced HBOT more than 4 h after the onset of symptoms. However, even within the 24 h included in this series, patients gained significant visual improvement with HBOT, perhaps CRAO in vivo is not as complete as central retinal artery clumping.
The limitations of this study are inherent to a retrospective study. First, patients in the HBOT group were significantly older compared to the patients in the control group (mean age 69 and 60, respectively). Also, the duration of symptoms was shorter in the HBOT group (9.1 vs. 19 h), perhaps the 24-h treatment window for HBOT set at our medical centre resulted in shorter referral times. The aim of the control group was to merely represent the natural history of VA changes with conservative treatment and thus the difference in the duration of symptoms at the presentation is less relevant. Moreover, after adjustment for both age and duration of symptoms, final BCVA was significantly better in the HBOT group.
Lens status and refractive errors of the patients were not recorded in this series and thus might differ between the groups. However, our analysis was based on VA changes within the groups and given that the VA did not differ significantly at baseline (P = 0.49) this is unlikely to affect the results.
In conclusion, it seems that HBOT for non-arteritic CRAO has superior visual outcomes compared to conventional treatment. Given that proportion of patients gaining functional vision was similar to the control group, the clinical and quality of life effects of such improvement and perhaps the cost-effectiveness of this treatment needs to be confirmed with further research.
Summary
What was known before
-
The aim of hyperbaric oxygen treatment in CRAO is to increase the oxygen delivery to the ischaemic tissue until spontaneous or assisted reperfusion occurs.
What this study adds
-
Hyperbaric oxygen treatment for non-arteritic CRAO has superior visual outcomes compared to conventional treatment.
-
The clinical and quality of life impacts of such improvement needs to be confirmed with further research.
References
Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152:820–3.e2.
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89.
Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125:1597–607.
Mueller AJ, Neubauer AS, Schaller U, Kampik A. Evaluation of minimally invasive therapies and rationale for a prospective randomized trial to evaluate selective intra-arterial lysis for clinically complete central retinal artery occlusion. Arch Ophthalmol (Chic, Ill: 1960). 2003;121:1377–81.
Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140:376–91.
Schumacher M, Schmidt D, Jurklies B, Gall C, Wanke I, Schmoor C, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117:1367–75.e1.
Garcia-Arumi J, Martinez-Castillo V, Boixadera A, Fonollosa A, Corcostegui B. Surgical embolus removal in retinal artery occlusion. Br J Ophthalmol. 2006;90:1252–5.
Takata Y, Nitta Y, Miyakoshi A, Hayashi A. Retinal endovascular surgery with tissue plasminogen activator injection for central retinal artery occlusion. Case Rep Ophthalmol. 2018;9:327–32.
Piantadosi CA. Physiology of hyperbaric hyperoxia. Respir Care Clin N Am. 1999;5:7–19.
Menzel-Severing J, Siekmann U, Weinberger A, Roessler G, Walter P, Mazinani B. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am J Ophthalmol. 2012;153:454–9.e2.
Hadanny A, Maliar A, Fishlev G, Bechor Y, Bergan J, Friedman M, et al. Reversibility of retinal ischemia due to central retinal artery occlusion by hyperbaric oxygen. Clin Ophthalmol. 2017;11:115–25.
Weinberger AW, Siekmann UP, Wolf S, Rossaint R, Kirchhof B, Schrage NF. [Treatment of acute central retinal artery occlusion (CRAO) by hyperbaric oxygenation therapy (HBO)—pilot study with 21 patients]. Klin Monatsblatter Augenheilkd. 2002;219:728–34.
Beiran I, Goldenberg I, Adir Y, Tamir A, Shupak A, Miller B. Early hyperbaric oxygen therapy for retinal artery occlusion. Eur J Ophthalmol. 2001;11:345–50.
Beiran I, Reissman P, Scharf J, Nahum Z, Miller B. Hyperbaric oxygenation combined with nifedipine treatment for recent-onset retinal artery occlusion. Eur J Ophthalmol. 1993;3:89–94.
Aisenbrey S, Krott R, Heller R, Krauss D, Rossler G, Heimann K. [Hyperbaric oxygen therapy in retinal artery occlusion]. Ophthalmologe: Z Dtsch Ophthalmologischen Ges. 2000;97:461–7.
Cope A, Eggert JV, O’Brien E. Retinal artery occlusion: visual outcome after treatment with hyperbaric oxygen. Diving Hyperb Med. 2011;41:135–8.
Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25.
Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Exp Eye Res. 2004;78:723–36.
Author information
Authors and Affiliations
Contributions
AR was responsible for designing the protocol, interpreting results and led the writing of the manuscript. AH was responsible for extracting the data and contributed to the writing of the final manuscript. AP was responsible for extracting the data. BD-P was responsible for analysing data and interpreting results. LO contributed to result interpretation and the writing of the final manuscript. EE, SE and EP contributed to the protocol design, interpretation of results and writing the final manuscript. AE-L devised and supervised the project and the main conceptual ideas, contributed to the final manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rozenberg, A., Hadad, A., Peled, A. et al. Hyperbaric oxygen treatment for non-arteritic central retinal artery occlusion retrospective comparative analysis from two tertiary medical centres. Eye 36, 1261–1265 (2022). https://doi.org/10.1038/s41433-021-01617-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01617-8
This article is cited by
-
Ocular effects of hyperbaric oxygen therapy
Eye (2024)
-
Retinale arterielle Verschlüsse (RAV)
Die Ophthalmologie (2023)