Abstract
Objective
The objective of this study was to assess the structural–functional relationship in choroideremia (CHM) patients using optical coherence tomography (OCT) and autofluorescence (AF) images.
Methods
In this study, 53 eyes of 28 CHM patients were included. Demographic, ocular and clinical fundus features were recorded. Fundus AF and OCT images were analysed. Patients were classified into two groups based on AF features: group 1, CHM patients where the foveal island was present and group 2, CHM patients where the foveal island was absent. Inner and outer retinal layer thicknesses, retinal pigment epithelium (RPE) and subfoveal choroidal thickness (SFCT) were measured and correlated with visual acuity (VA).
Results
There were 26 eyes in group 1 and 27 eyes in group 2. Mean age in groups 1 and 2 were 51.7 ± 13.4 and 63.6 ± 11.6 years, respectively. Age (p = 0.001) and VA (p < 0.001) between the two groups were significantly different. The retinal and SFCT showed significant differences that were analysed for each eye between the two groups. Reduced VA was noted with increasing age (r = 0.483; p ≤ 0.001), thin total retina (r = −0.378; p = 0.005), inner (r = −0.512; p < 0.001), outer (r = −0.59; p < 0.001) retinal thicknesses and thin RPE (r = −0.653; p < 0.001). Multivariate analysis showed RPE thickness (p = 0.001) as the most important index that affected VA.
Conclusion
RPE thinning contributes to poor VA in patients with advanced CHM. Further studies are needed to evaluate the role of retinal thickness and SFCT and its relationship to VA.
Similar content being viewed by others
Introduction
Choroideremia (CHM) is an X-linked recessive condition that primarily affects males. It is characterised by night blindness in early childhood, progressive constriction of peripheral fields, vision loss and finally blindness in late adulthood [1]. It is caused by mutations in the CHM gene, which codes for REP-1 (Rab-escort protein-1) [2, 3]. The lack of functional REP-1 prevents Rab proteins from reaching and binding to the organelle membranes, as a result of which the cells of the photoreceptor layer, retinal pigment epithelium (RPE) layer and choroid/choriocapillaris die prematurely. This gives a characteristic atrophic fundus appearance. It is still not very clear whether the loss of each layer occurs sequentially due to interdependent cell survival, or independently. This question has now become clinically relevant as it would determine which layer could be the primary target for gene replacement therapy for CHM.
Fundus autofluorescence (AF) imaging, near-infrared AF imaging and visual field testing are routinely used to monitor the progression in CHM [4,5,6,7]. Currently, blue AF (B-AF) imaging, using a 488-nm laser excitation light, is most frequently used in clinical practice [4, 5]. B-AF imaging in CHM has shown that centripetal RPE degeneration occurs along with early loss of peripheral AF leading to the formation of a small residual ‘island’ of preserved AF with sharply demarcated scalloped edges, usually centred around the fovea [4]. This island shrinks over time and eventually encroaches the fovea, resulting in a sharp decline of visual acuity generally during the fifth decade of life [4]. Changes in the size of the AF island over time could be used to monitor disease progression [5]. However, it is unclear how the changes in AF, which is a marker for RPE health, correlate with the state of the photoreceptors and choroid/choriocapillaris and visual acuity.
Functional changes in CHM, mainly visual acuity, is a result of the structural changes in the retina, RPE and choroidal layers at the fovea seen on optical coherence tomography (OCT) and the extent of residual island of preserved AF. Xue et al. [5] studied the relationship between RPE, photoreceptor, and choroidal degeneration in CHM. They found a correlation between the quality of AF and integrity of ellipsoid zone within islands of the surviving retina. They showed reduced subfoveal choroidal thickness (SFCT) in eyes with CHM compared to controls, but it did not undergo significant thinning until end-stage retinal degeneration. However, in this study, the correlation of preserved island on AF with the visual acuity was not evaluated. A study by Heon et al. [6] has shown thinner central retinal thickness (CRT) to be associated with poor visual acuity. However, there is very limited literature correlating the inner and outer retinal layers thicknesses, RPE and the choroid with visual acuity [5, 6]. Also, in eyes with advanced CHM, identified by the loss of the island of preserved AF, the contribution of the choroid and/or photoreceptors towards the visual acuity needs to be investigated.
The recent success of gene therapy in the treatment of CHM has generated a lot of hope and enthusiasm in the management of this condition [8,9,10,11]. However, in order to achieve desirable results, a proper selection of candidates for gene therapy is a must.
With this background, the primary aim of this study was to assess the structural–functional relationship in CHM patients by correlating the structural changes visible on OCT and AF images with visual acuity. Specifically, the thickness of the different retinal layers and the choroid in patients with and without advanced CHM and its correlation with visual acuity was investigated. A better understanding of the changes in the structural and functional features of the retina in CHM would help in identifying the right candidate for gene replacement therapy.
Methods
This work was approved by our Institutional Review Board and ethics committee (C/2020/07/009) and respected the tenets of the Declaration of Helsinki. In this retrospective non-interventional image analysis of enhanced depth imaging OCT (EDI-OCT) and AF images, patients of CHM identified through our internal database from July 2015 to June 2020 were included in the study. The study was carried out at the Narayana Nethralaya super speciality eye hospital, Bangalore, India. The diagnosis of CHM was suspected in a patient presenting with characteristic symptoms of nyctalopia and progressively reduced peripheral vision and fundus showing peripheral pigmentary changes and chorioretinal atrophy, which later involve the posterior pole and peripapillary region (Fig. 1) [12]. Inclusion criteria were male patients with either straightforward clinical diagnosis of CHM with or without molecular diagnosis of CHM when possible. Cases who presented with early-onset night blindness, rapid deterioration to legal blindness and presence of bony spicules, arteriolar attenuation and waxy disc pallor were clinically diagnosed as X-linked retinitis pigmentosa (XLRP) and were excluded [13]. Also, cases of XLRP confirmed on genetic testing were excluded from the study.
Genetic testing
After obtaining the detailed family and pedigree history, 5 ml of blood was withdrawn to isolate the DNA by salt precipitation method [14]. Targeted next-generation sequencing was carried out using a method described previously, which included sample preparation, target enrichment, exome sequencing data analysis and variant calling [15]. For the variant interpretation, CHM (for choroideremia) and RPGR and RP2 genes (for XLRP) were included for the annotation, prioritisation and reporting following the ACMG (American College of Medical Genetics and Genomics) guidelines. The PCR products were sequenced (3500 DX, Lifetech Inc., USA) by Sanger sequencing method. The sequence electropherogram was visualised using the FinchTV software (Geospiza, USA), and the presence of the variation in genes was evaluated by comparing the patient’s sequence with the reference sequence.
The medical records of all the eligible patients were reviewed and the following data were collected: age, gender, involved eye, best-corrected visual acuity, refractive error, axial length and clinical features on retinal examination. The fundus features of all the patients were documented with ultra-widefield colour fundus photograph using the Optos Optomap Daytona Panoramic 200Tx (Daytona, Optos®, UK). A blue-wavelength laser fundus AF 30° or 55° image centred at the fovea was captured according to the manufacturer’s standard operating procedure. The macular AF image was analysed to check for a residual island of preserved AF with sharply demarcated scalloped edges, usually centred around the fovea. In case the fovea was not clearly visualised, the brightness and contrast settings of the image were adjusted to correctly identify the optic disc and then the exact location of the macula would be ascertained.
In the same sitting, a high-resolution spectral-domain OCT was obtained using the Spectralis HRA OCT system (Heidelberg Engineering, Heidelberg, Germany) with 25-line horizontal volume scans covering the area centred over the fovea. EDI mode was activated during OCT capture and a simultaneous infrared confocal scanning laser ophthalmoscope image was captured automatically. Outer retinal tubulations over the areas of degeneration if present were noted. Quantitative measurements regarding the CRT and SFCT were recorded using the automated measurement tool with the proprietary machine software in the Spectralis machine.
Image analysis
AF analysis
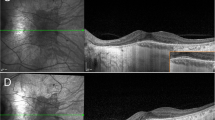
Based on the identification of the features on AF by a single observer (JPC), patients were classified into two groups: group 1, CHM patients where the foveal island of preserved AF was present and group 2, CHM patients where the foveal island of preserved AF was absent (advanced CHM) (Fig. 2).
A, B Ultrawide fundus images acquired using the Optos, Daytona machine showing an atrophic fundus due to the diffuse degeneration of the photoreceptors, retinal pigment epithelium and choroidal layer. C, D The 55° blue-wavelength fundus autofluorescence images of both eyes showing a small residual island of preserved autofluorescence at the macula. Optical coherence tomography images of both eyes through the macula in CHM.
Retinal analysis
All thickness measurements were made on the SD-OCT using the automated layer segmentation software. In cases of automatic layer misalignment, manual alignment was possible by the SD-OCT software before automatic measurements. A semiautomated approach was incorporated into the algorithm to correct for any minor segmentation errors. In addition, all of the boundaries were checked by visual inspection performed by two of the authors (RV and NR). For evaluation of the macular area, each macular thickness map was divided into nine regions suggested by the Early Treatment for Diabetic Retinopathy Study including a 1-mm-diameter central disc and an inner and outer ring, each divided into four quadrants, with diameters of 3 and 6 mm, respectively [16]. OCT delineates every macular layer, and we measured the thickness of inner retinal layer (from the internal limiting membrane to external limiting membrane), outer retinal layer (from external limiting membrane to RPE layer), RPE layer alone and total CRT (from the internal limiting membrane to RPE layer) at the 1-mm-diameter central disc as suggested by the Early Treatment for Diabetic Retinopathy Study [16]. All these measurements were automatically obtained using the in-built software in the Spectralis SD-OCT machine (Fig. 3).
Choroidal analysis
A single-line horizontal raster scan (30° wide) using the EDI mode extending from the temporal margin of the optic nerve head and passing through the foveal centre was used to measure the choroidal thickness. The SFCT was measured from the outer border of the RPE to the inner border of the sclera under the fovea.
Main outcome measures
-
(1)
To compare the demographic, clinical and OCT features between the two groups.
-
(2)
To identify the factors on OCT affecting the visual acuity of CHM patients.
Statistical analysis
All data were analysed with the GraphPad Prism software (version 8.4.2 [676] for Windows), San Diego, CA. The Kolmogorov–Smirnov normality test showed the data sets to be of the non-parametric variety and hence only non-parametric statistical tests were used in this study. Visual acuity data were converted to logMAR for statistical analysis. For continuous data, Mann–Whitney U test was used to compare the findings for each eye separately between the two groups. Contingency analysis was done using the χ2 test for categorical data between two groups. Correlation between the age, retinal and choroidal thicknesses and final visual acuity were analysed using the Spearman’s correlation test. Multivariate linear regression analyses were performed between the visual acuity as the dependent variable and age and retinal and choroidal thicknesses as independent variables. A generalised estimating equation model for both eyes was used for estimating the average response over the population to compensate for both eyes’ inclusion of subjects. P values < 0.05 were considered statistically significant.
Results
In this study, 53 eyes of 28 male subjects who met the inclusion criteria were included. There were 26 eyes of 14 patients who had an island of preserved AF at the macula (group 1) and 27 eyes of 14 patients who had no island of preserved AF at the macula (group 2). One eye each having poor media clarity due to mature cataract, corneal scar and phthisis bulbi, respectively, and not allowing detailed retinal examination were excluded from the study. Two patients of group 2 in the study belonged to the same family. The demographic and ocular features noted at the time of examination in the two groups were analysed, compared and described in Table 1.
The mean age of the participants in groups 1 and 2 were 51.7 ± 13.4 and 63.6 ± 11.6 years, respectively. The retinal and choroidal thickness was measured for each eye separately between the two groups and were further compared, analysed and described in Table 2. Between the two groups, age (p = 0.001) and visual acuity (p < 0.001) were significantly different. Spherical equivalent (p = 0.518) and axial length (p = 0.333) were comparable and not statistically significant. Outer retinal tubulations were identified in 18 (69%) and 23 (85%) eyes in groups 1 and 2, respectively. The CRT and SFCT between the two groups were significantly different in the right eye and left eye, respectively.
Table 3 describes the correlation of the various indices that could affect the final visual acuity using the Spearman’s correlation test. Decline in visual acuity was noted with increasing age (r = 0.483; p ≤ 0.001), low CRT (r = −0.378; p = 0.005), thin IRL (r = −0.512; p < 0.001), thin ORL (r = −0.59; p < 0.001) and thin RPE (r = −0.653; p < 0.001). However, multivariate linear regression analysis of these significant variables identified the thickness of the RPE as the single most important index which affects the visual acuity (Table 4).
Discussion
The results of this study suggest that there is a significant thinning of all the retinal layers and choroid in eyes with advanced CHM. Multivariate regression analysis identified the RPE thinning solely responsible for predicting the visual acuity in eyes with CHM.
In CHM, there is a gradual progressive degeneration of the RPE, photoreceptors and choroid leading to visual loss and finally blindness. However, the etiopathogenesis of CHM is poorly understood. Whether the RPE or photoreceptors or choroid gets affected first in CHM is not well known. Animal models have been studied to elucidate the pathogenesis of CHM. Studies in mice by Tolmachova et al. [17] have suggested that CHM pathogenesis involves independently triggered degeneration of photoreceptors and the RPE, associated with different subsets of defective Rabs. A few years later, the same group suggested that the RPE loss accelerates the photoreceptor degeneration in cell-type-specific knockout mouse models of CHM [18].
The disease expression in the human CHM retinae is more complex. Early stages show a thickening of the retina that was otherwise normally laminated. Further, there is loss of photoreceptors, either independent or associated with RPE depigmentation, followed by disorganisation and further thickening of the retina with interlaminar bridges. Later, the dysmorphic retina undergoes thinning over decades. Laminopathy affects the more peripheral rod-rich regions first and later involves the cones at the fovea [19]. Even in the present study, we found the retina to be thicker in less advanced CHM cases compared to cases that had advanced CHM. The thickened retina in the early stages could be explained due to the Müller cell activation and hypertrophy from photoreceptor stress as suggested by Jacobson et al. [19]. In advanced cases of CHM, choroidal thinning was noted in our study. Similar atrophy of the choroid and choriocapillaris layers were noted in CHM patients compared to normal controls [5]. The choroid does not undergo significant thinning until end-stage retinal degeneration develops. This suggests that choroidal degeneration identified by the choroidal thinning may be a secondary phenomenon, which lags significantly behind photoreceptor and RPE degeneration. Another observation from this study was the thinning of RPE and outer retinal layers in cases of advanced CHM. This finding is in sync with other studies that report RPE loss or atrophy and photoreceptor thinning in advanced cases of CHM [5, 6, 19]. Also, the authors noted that the atrophied RPE in advanced cases correlated with poor visual acuity.
The ellipsoid zone and outer retinal integrity are strongly linked to visual prognosis [20]. A study by Arepalli et al. [21] evaluating the thickness of ellipsoid zone, outer retina and inner retina in eyes without macular disease across a wide age spectrum suggested that thinning of the inner and outer retinal layers was associated with reduced visual acuity. One of the important functions of the RPE is to provide nutritional and metabolic support to the choroid and photoreceptors [22]. Thinning of the RPE results in secondary degeneration of the photoreceptors and choroid/choriocapillaris, thereby affecting the visual acuity. Further studies would be required to confirm the author’s observation. The structural and vascular changes in the retina and choroid due to CHM can have a bearing on the visual acuity. In the present study, a decline in visual acuity correlated with thinning of the retinal, choroidal and RPE layers.
The global prevalence of CHM ranges from 1:50,000 to 1:100,000 [23]. The current study showed a high diagnosis rate of CHM in the hospital population. One could argue that this could be due to the phenomenon of ‘founder effect’ and genetic drift as reported in Finland and Canada [24]. In ‘founder effect’, the affected allele survives and within a few generations becomes much more dispersed throughout the population. The new population experiences an increase in the frequency of recessive alleles, as well, and as a result, an increased number who are homozygous for certain recessive traits. The phenomenon of ‘founder effect’ could be established when multiple cases of CHM (either affected or carriers) are identified in the same family, caste, restricted demographic group or societies practising endogamy. However, in this study, there were only two patients diagnosed with CHM who belonged to the same family. In addition, our hospital being a tertiary centre in Bangalore receives patients referred from other centres within the city, state and country. Hence, we believe that the ‘founder effect’ would not have played an important role in the high number of cases of CHM in our study.
This study has many limitations; first, molecular diagnosis of CHM was not available for all cases (only in 5 of the 28 patients). Also, as there is a phenotypic overlap of the clinical features of CHM and XLRP, there could be a possible misdiagnosis of CHM in some cases. Second, female carriers were not included and hence the structural and functional correlation in these patients were not studied. In female carriers of CHM, their cone photoreceptor densities and SFCT are well preserved compared to the male probands [5, 25]. These observations may account for the good visual acuity and lack of an awareness of visual disturbances. Third, the OCT angiography details were not available to assess the choriocapillaris damage. Choroidal thickness alone does not reflect choriocapillaris loss and thus it would not be possible to comment whether the RPE damage is more in comparison to choriocapillaris damage.
In conclusion, RPE is primarily affected in CHM, which correlates with the photoreceptor and choroidal/choriocapillaris degeneration; a finding that may have implications on future gene therapies. The RPE thinning is one of the major contributors to the poor visual acuity in patients with advanced CHM. Larger studies are required to further evaluate the role of the retinal and choroidal thickness and its relationship to visual acuity in CHM using more sophisticated imaging modalities such as OCT angiography or adaptive optics.
Summary
What was known before
-
CHM is an X-linked recessive condition that primarily affects males. It is characterised by night blindness in early childhood, progressive constriction of peripheral fields, vision loss and finally blindness in late adulthood.
-
The cells of the photoreceptor layer, retinal pigment epithelium layer and choroid/choriocapillaris die prematurely. It is still not very clear whether the loss of each layer occurs sequentially due to interdependent cell survival, or independently.
-
In CHM, there is thinning of the CRT and choroidal thickness compared to the controls.
-
What contributes to the poor vision in advanced CHM is not very clear.
What this study adds
-
RPE is primarily affected in CHM, which correlates with the photoreceptor and choroidal/choriocapillaris degeneration; a finding that may have implications on future gene therapies.
-
The RPE thinning is one of the major contributors to the poor visual acuity in patients with advanced CHM.
-
Study of retinal structural–functional relationship in choroideremia using fundus AF and OCT.
References
MacDonald IM, Russell L, Chan C-C. Choroideremia: new findings from ocular pathology and review of recent literature. Surv Ophthalmol. 2009;54:401–7.
van den Hurk JA, Schwartz M, van Bokhoven H, van de Pol TJ, Bogerd L, Pinckers AJ, et al. Molecular basis of choroideremia (CHM): mutations involving the Rab escort protein-1 (REP-1) gene. Hum Mutat. 1997;9:110–7.
van den Hurk JAJM, van de Pol DJR, Wissinger B, van Driel MA, Hoefsloot LH, de Wijs IJ, et al. Novel types of mutation in the choroideremia (CHM) gene: a full-length L1 insertion and an intronic mutation activating a cryptic exon. Hum Genet. 2003;113:268–75.
Jolly JK, Edwards TL, Moules J, Groppe M, Downes SM, MacLaren RE. A qualitative and quantitative assessment of fundus autofluorescence patterns in patients with choroideremia. Invest Ophthalmol Vis Sci. 2016;57:4498–503.
Xue K, Oldani M, Jolly JK, Edwards TL, Groppe M, Downes SM, et al. Correlation of optical coherence tomography and autofluorescence in the outer retina and choroid of patients with choroideremia. Invest Ophthalmol Vis Sci. 2016;57:3674–84.
Heon E, Alabduljalil T, McGuigan DB III, Cideciyan AV, Li S, Chen S, et al. Visual function and central retinal structure in choroideremia. Invest Ophthalmol Vis Sci. 2016;57:OCT377–387.
Birtel J, Salvetti AP, Jolly JK, Xue K, Gliem M, Müller PL, et al. Near-infrared autofluorescence in choroideremia: anatomic and functional correlations. Am J Ophthalmol. 2019;199:19–27.
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37.
Xue K, MacLaren RE. Ocular gene therapy for choroideremia: clinical trials and future perspectives. Expert Rev Ophthalmol. 2018;13:129–38.
Barnard AR, Groppe M, MacLaren RE. Gene therapy for choroideremia using an adeno-associated viral (AAV) vector. Cold Spring Harb Perspect Med. 2014;5:a017293.
Anand V, Barral DC, Zeng Y, Brunsmann F, Maguire AM, Seabra MC, et al. Gene therapy for choroideremia: in vitro rescue mediated by recombinant adenovirus. Vis Res. 2003;43:919–26.
Coussa RG, Kim J, Traboulsi EI. Choroideremia: effect of age on visual acuity in patients and female carriers. Ophthalmic Genet. 2012;33:66–73.
Fishman GA, Farber MD, Derlacki DJ. X-linked retinitis pigmentosa. Profile of clinical findings. Arch Ophthalmol. 1988;106:369–75.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Battu R, Jeyabalan N, Murthy P, Reddy K, Schouten J, Webers C. Genetic analysis and clinical phenotype of two Indian families with X-linked choroideremia. Indian J Ophthalmol. 2016;64:924.
Anon. Early treatment diabetic retinopathy study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98 Suppl:741–56.
Tolmachova T, Anders R, Abrink M, Bugeon L, Dallman MJ, Futter CE, et al. Independent degeneration of photoreceptors and retinal pigment epithelium in conditional knockout mouse models of choroideremia. J Clin Invest. 2006;116:386–94.
Tolmachova T, Wavre-Shapton ST, Barnard AR, MacLaren RE, Futter CE, Seabra MC. Retinal pigment epithelium defects accelerate photoreceptor degeneration in cell type-specific knockout mouse models of choroideremia. Invest Ophthalmol Vis Sci. 2010;51:4913–20.
Jacobson SG, Cideciyan AV, Sumaroka A, Aleman TS, Schwartz SB, Windsor EAM, et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47:4113–20.
Itoh Y, Vasanji A, Ehlers JP. Volumetric ellipsoid zone mapping for enhanced visualisation of outer retinal integrity with optical coherence tomography. Br J Ophthalmol. 2016;100:295–9.
Arepalli S, Srivastava SK, Hu M, Kaiser PM, Dukles N, Reese JL, et al. Assessment of inner and outer retinal layer metrics on the Cirrus HD-OCT Platform in normal eyes. PLoS ONE. 2018;13:e0203324.
Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye. 2001;15 Part 3:384–9.
Coussa RG, Traboulsi EI. Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet. 2012;33:57–65.
O’Brien E, Jorde LB, Rönnlöf B, Fellman JO, Eriksson AW. Founder effect and genetic disease in Sottunga, Finland. Am J Phys Anthropol. 1988;77:335–46.
Suzuki K, Gocho K, Akeo K, Kikuchi S, Kubota D, Katagiri S. et al. High-resolution retinal imaging reveals preserved cone photoreceptor density and choroidal thickness in female carriers of choroideremia. Ophthalmic Surg Lasers Imaging Retina. 2019;50:76–85.
Author information
Authors and Affiliations
Contributions
RV, JC—conceptualising the study, data acquisition, analysing the data, interpreting the findings, and writing and reviewing the manuscript. NR, RS, JPC, AS—data acquisition and analysing the data. AP, RS, PRN, NR—analysing the data, interpreting the findings and reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Venkatesh, R., Reddy, N.G., Snehith, R. et al. Study of retinal structural–functional relationship in choroideremia using fundus autofluorescence and optical coherence tomography. Eye 35, 3389–3396 (2021). https://doi.org/10.1038/s41433-021-01441-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01441-0