Abstract
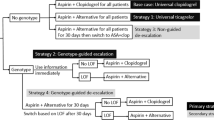
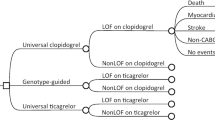
CYP2C19 genotyping to guide antiplatelet therapy after patients develop acute coronary syndromes (ACS) or require percutaneous coronary interventions (PCIs) reduces the likelihood of major adverse cardiovascular events (MACE). Evidence about the impact of preemptive testing, where genotyping occurs while patients are healthy, is lacking. In patients initiating antiplatelet therapy for ACS or PCI, we compared medical records data from 67 patients who received CYP2C19 genotyping preemptively (results >7 days before need), against medical records data from 67 propensity score-matched patients who received early genotyping (results within 7 days of need). We also examined data from 140 patients who received late genotyping (results >7 days after need). We compared the impact of genotyping approaches on medication selections, specialty visits, MACE and bleeding events over 1 year. Patients with CYP2C19 loss-of-function alleles were less likely to be initiated on clopidogrel if they received preemptive rather than early or late genotyping (18.2%, 66.7%, and 73.2% respectively, p = 0.001). No differences were observed by genotyping approach in the number of specialty visits or likelihood of MACE or bleeding events (all p > 0.21). Preemptive genotyping had a strong impact on initial antiplatelet selection and a comparable impact on patient outcomes and healthcare utilization, compared to genotyping ordered after a need for antiplatelet therapy had been identified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study.
References
Lee CR, Luzum JA, Sangkuhl K, Gammal RS, Sabatine MS, Stein CM, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharm Ther. 2022;112:959–67.
McDermott JH, Leach M, Sen D, Smith CJ, Newman WG, Bath PM. The role of CYP2C19 genotyping to guide antiplatelet therapy following ischemic stroke or transient ischemic attack. Expert Rev Clin Pharm. 2022;15:811–25.
Ellithi M, Baye J, Wilke RA. CYP2C19 genotype-guided antiplatelet therapy: promises and pitfalls. Pharmacogenomics. 2020;21:889–97.
Essa M, Ross JS, Dhruva SS, Desai NR, Yeh RW, Faridi KF. Trends in spending and claims for P2Y12 inhibitors by medicare and medicaid from 2015 to 2020. J Am Heart Assoc. 2023;12:e028869.
Danielak D, Pawlak K, Główka F, Karaźniewicz-Łada M. Influence of genetic and epigenetic factors of P2Y(12) receptor on the safety and efficacy of antiplatelet drugs. Cardiovasc Drugs Ther. 2022. https://doi.org/10.1007/s10557-022-07370-8.
Limdi NA, Cavallari LH, Lee CR, Hillegass WB, Holmes AM, Skaar TC, et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharmacogenomics J. 2020;20:724–35.
Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Inter. 2018;11:181–91.
Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 2020;324:761–71.
Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N. Engl J Med. 2019;381:1621–31.
Yoon HY, Lee N, Seong JM, Gwak HS. Efficacy and safety of clopidogrel versus prasugrel and ticagrelor for coronary artery disease treatment in patients with CYP2C19 LoF alleles: a systemic review and meta-analysis. Br J Clin Pharm. 2020;86:1489–98.
Malik AH, Gupta R, Chakraborty S, Mahajan P, Bandyopadhyay D, Yandrapalli S, et al. Effect of genotype-guided oral P2Y12 inhibitor selection after percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Cardiovasc Revasc Med. 2022;41:115–21.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–60.
Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–8.
Galli M, Benenati S, Franchi F, Rollini F, Capodanno D, Biondi-Zoccai G, et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61,898 patients from 15 randomized trials. Eur Heart J. 2022;43:959–67.
U.S. Food and Drug Administration. FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. 2010. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-reduced-effectiveness-plavix-clopidogrel-patients-who-are-poor.
Roden DM, Van Driest SL, Mosley JD, Wells QS, Robinson JR, Denny JC, et al. Benefit of preemptive pharmacogenetic information on clinical outcome. Clin Pharm Ther. 2018;103:787–94.
Ji Y, Skierka JM, Blommel JH, Moore BE, VanCuyk DL, Bruflat JK, et al. Preemptive pharmacogenomic testing for precision medicine: a comprehensive analysis of five actionable pharmacogenomic genes using next-generation DNA sequencing and a customized CYP2D6 genotyping cascade. J Mol Diagn. 2016;18:438–45.
Weitzel KW, Cavallari LH, Lesko LJ. Preemptive panel-based pharmacogenetic testing: the time is now. Pharm Res. 2017;34:1551–5.
Petry NJ, Curtis B, Feldhege E, Khan S, Leedahl DD, Breidenbach JL, et al. Impact of automated best practice advisories on provider response to CYP2C19 genotyping results for patients on clopidogrel. J Pharm Pr. 2023;36:487–93. 2021:8971900211049589
O’Donnell PH, Wadhwa N, Danahey K, Borden BA, Lee SM, Hall JP, et al. Pharmacogenomics-based point-of-care clinical decision support significantly alters drug prescribing. Clin Pharm Ther. 2017;102:859–69.
Peterson JF, Field JR, Unertl KM, Schildcrout JS, Johnson DC, Shi Y, et al. Physician response to implementation of genotype-tailored antiplatelet therapy. Clin Pharm Ther. 2016;100:67–74.
Weitzel KW, Elsey AR, Langaee TY, Burkley B, Nessl DR, Obeng AO, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166c:56–67.
Luzum JA, Pakyz RE, Elsey AR, Haidar CE, Peterson JF, Whirl-Carrillo M, et al. The pharmacogenomics research network translational pharmacogenetics program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin Pharm Ther. 2017;102:502–10.
van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Dávila-Fajardo CL, Deneer VH, et al. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharm Ther. 2017;101:341–58.
Swen JJ, van der Wouden CH, Manson LE, Abdullah-Koolmees H, Blagec K, Blagus T, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401:347–56.
Food and Drug Administration. FDA issues warning letter to genomics lab for illegally marketing genetic test that claims to predict patients’ responses to specific medications. 2018. https://www.fda.gov/news-events/press-announcements/fda-issues-warning-letter-genomics-lab-illegally-marketing-genetic-test-claims-predict-patients.
Petry N, Baye J, Aifaoui A, Wilke RA, Lupu RA, Savageau J, et al. Implementation of wide-scale pharmacogenetic testing in primary care. Pharmacogenomics. 2019;20:903–13.
Christensen KD, Bell M, Zawatsky CLB, Galbraith LN, Green RC, Hutchinson AM, et al. Precision population medicine in primary care: the Sanford Chip experience. Front Genet. 2021;12:626845.
Moyer AM, Rohrer Vitek CR, Giri J, Caraballo PJ. Challenges in ordering and interpreting pharmacogenomic tests in clinical practice. Am J Med. 2017;130:1342–4.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47.
Hajek C, Hutchinson AM, Galbraith LN, Green RC, Murray MF, Petry N, et al. Improved provider preparedness through an 8-part genetics and genomic education program. Genet Med. 2022;24:214–24.
Blout Zawatsky CL, Leonhard JR, Bell M, Moore MM, Petry NJ, Platt DM, et al. Workforce considerations when building a precision medicine program. J Pers Med. 2022;12:1929.
Martin J, Williams AK, Klein MD, Sriramoju VB, Madan S, Rossi JS, et al. Frequency and clinical outcomes of CYP2C19 genotype-guided escalation and de-escalation of antiplatelet therapy in a real-world clinical setting. Genet Med. 2020;22:160–9.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl J Med. 2007;357:2001–15.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl J Med. 2009;361:1045–57.
Chan NC, Eikelboom JW, Ginsberg JS, Lauw MN, Vanassche T, Weitz JI, et al. Role of phenotypic and genetic testing in managing clopidogrel therapy. Blood. 2014;124:689–99.
Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72:2915–31.
Kalow W, Tang BK, Endrenyi L. Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283–9.
Belle DJ, Singh H. Genetic factors in drug metabolism. Am Fam Physician. 2008;77:1553–60.
Brunette CA, Dong OM, Vassy JL, Danowski ME, Alexander N, Antwi AA, et al. A cost-consequence analysis of preemptive SLCO1B1 testing for statin myopathy risk compared to usual care. J Pers Med. 2021;11:1123.
Acknowledgements
Members of the Imagenetics METRICS TEAM. Sanford Health: Jordan Baye, Colette Free, Catherine Hajek, Kristen Jacobsen, Mary Kara, Amanda Massmann, Jennifer Morgan, Marian Petrasko, Natasha Petry, Muhammad Hamza Saad Shaukat, April Schultz, Rebecca Scott, Garret Spindler, Adam Stys, Tomasz Stys, Joel Van Heukelom, Max Weaver, and Elizabeth Wheeler. Harvard Pilgrim Health Care Institute: Kurt Christensen, Lauren Galbraith, Madison Hickingbotham, Jessica LeBlanc, and Emilie Zoltick. Brigham and Women’s Hospital: Robert Green, Charlene Preys and Carrie Zawatsky. National Institutes of Health: Leila Jamal.
Funding
This work was supported by the National Institutes of Health [K01-HG009173 to KDC] and the Imagenetics Initiative and Sanford Chip Program from Sanford Health.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.M., J.V.H., R.C.G., C.H., M.R.H., E.S.Z., K.D.C., A.J.S.; Data curation: A.M., J.V.H., M.R.H., K.D.C., A.J.S.; Formal analysis: M.R.H., K.D.C. M.W.; Funding acquisition: R.C.G., C.H., K.D.C., A.J.S.; Investigation: A.M., J.V.H., M.R.H., E.S.Z., K.D.C., A.J.S., M.H.S., A.S., T.S.; Methodology: A.M., J.V.H., M.R.H., A.C.W., E.S.Z., K.D.C., A.J.S., M.H.S., A.S., T.S.; Project administration: C.H., M.R.H., A.J.S.; Supervision: K.D.C., A.J.S.; Validation: A.M., J.V.H.; Writing-original draft: A.M., J.V.H., M.R.H., E.S.Z., K.D.C., A.J.S.; Writing–review & editing: A.M., J.V.H., R.C.G., C.H., M.R.H., E.A.L, A.C.W., E.S.Z., K.D.C., A.J.S., M.W., M.H.S., A.S., T.S.
Corresponding author
Ethics declarations
Competing interests
KDC, ESZ, and MRH were supported by a research grant from Sanford Health. CH is an employee of Helix OpCo, LLC. RCG has received compensation for advising Allelica, Atria, Fabric, Genome Web, Genomic Life, and VinBigData and is co-founder of Genome Medical and Nurture Genomics. The remaining authors have nothing to disclose.
Ethics
The research protocol was approved by the Sanford Health and Harvard Pilgrim Health Care Institute Institutional Review Boards. Individual-level patient data were deidentified before being provided to the study team, and informed consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Massmann, A., Christensen, K.D., Van Heukelom, J. et al. Clinical impact of preemptive pharmacogenomic testing on antiplatelet therapy in a real-world setting. Eur J Hum Genet (2024). https://doi.org/10.1038/s41431-024-01567-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41431-024-01567-1