Abstract
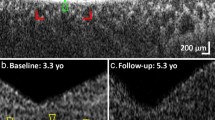
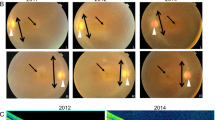
Intellectual disability (ID) and retinal dystrophy (RD) are the frequently found features of multiple syndromes involving additional systemic manifestations. Here, we studied a family with four members presenting severe ID and retinitis pigmentosa (RP). Using genome wide genotyping and exome sequencing, we identified a nonsense variant c.747 C > A (p.Tyr249Ter) in exon 7 of AGPAT3 which co-segregates with the disease phenotype. Western blot analysis of overexpressed WT and mutant AGPAT3 in HEK293T cells showed the absence of AGPAT3, suggesting instability of the truncated protein. Knockdown of Agpat3 in the embryonic mouse brain caused marked deficits in neuronal migration, strongly suggesting that reduced expression of AGPAT3 affects neuronal function. Altogether, our data indicates that AGPAT3 activity is essential for neuronal functioning and loss of its activity probably causes intellectual disability and retinitis pigmentosa (IDRP) syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data generated during the study are available from corresponding author on reasonable request. The variant information has been deposited in Clinvar with submission ID SCV 003929458.
References
Bee YM, Chawla M, Zhao Y. Whole exome sequencing identifies a novel and a recurrent mutation in BBS2 gene in a family with Bardet-Biedl syndrome. BioMed Res Int. 2015;2015:524754.
Novas R, Cardenas-Rodriguez M, Irigoín F, Badano JL. Bardet-Biedl syndrome: Is it only cilia dysfunction? FEBS Lett. 2015;589:3479–91.
Usta M, Urganci N, Özçelik G, Çetinçelik Ü, Kafadar I, Özgüven BY. Joubert syndrome and related disorders: a rare cause of intrahepatic portal hypertension in childhood. Eur Rev Med Pharmacol Sci. 2015;19:2297–300.
Cohen MM Jr, Hall BD, Smith DW, Graham CB, Lampert KJ. A new syndrome with hypotonia, obesity, mental deficiency, and facial, oral, ocular, and limb anomalies. J Pediatr. 1973;83:280–4.
Ronquillo CC, Bernstein PS, Baehr W. Senior-Løken syndrome: a syndromic form of retinal dystrophy associated with nephronophthisis. Vis Res. 2012;75:88–97.
Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38:191–6.
Yang XR, Benson MD, MacDonald IM, Innes AM. A diagnostic approach to syndromic retinal dystrophies with intellectual disability. Am J Med Genet Part C, Semin Med Genet. 2020;184:538–70.
Tatour Y, Sanchez-Navarro I, Chervinsky E, Hakonarson H, Gawi H, Tahsin-Swafiri S, et al. Mutations in SCAPER cause autosomal recessive retinitis pigmentosa with intellectual disability. J Med Genet. 2017;54:698–704.
Fasham J, Arno G, Lin S, Xu M, Carss KJ, Hull S, et al. Delineating the expanding phenotype associated with SCAPER gene mutation. Am J Med Genet Part A. 2019;179:1665–71.
Wormser O, Gradstein L, Yogev Y, Perez Y, Kadir R, Goliand I, et al. SCAPER localizes to primary cilia and its mutation affects cilia length, causing Bardet-Biedl syndrome. Eur J Hum Genet : EJHG. 2019;27:928–40.
Coppieters F, Ascari G, Dannhausen K, Nikopoulos K, Peelman F, Karlstetter M, et al. Isolated and syndromic retinal dystrophy caused by biallelic mutations in RCBTB1, a gene implicated in ubiquitination. Am J Hum Genet. 2016;99:470–80.
Di Donato N, Neuhann T, Kahlert AK, Klink B, Hackmann K, Neuhann I, et al. Mutations in EXOSC2 are associated with a novel syndrome characterised by retinitis pigmentosa, progressive hearing loss, premature ageing, short stature, mild intellectual disability and distinctive gestalt. J Med Genet. 2016;53:419–25.
Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–50.
van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol cell Biol. 2008;9:112–24.
Yamashita A, Hayashi Y, Nemoto-Sasaki Y, Ito M, Oka S, Tanikawa T, et al. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog lipid Res. 2014;53:18–81.
Agarwal AK. Lysophospholipid acyltransferases: 1-acylglycerol-3-phosphate o-acyltransferases. from discovery to disease. Curr Opin Lipidol. 2012;23:290–302.
Prasad SS, Garg A, Agarwal AK. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: localization of AGPAT5 to mitochondria. J lipid Res. 2011;52:451–62.
Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J cell Biol. 2009;186:211–8.
Schmidt JA, Yvone GM, Brown WJ. Membrane topology of human AGPAT3 (LPAAT3). Biochem Biophys Res Commun. 2010;397:661–7.
Yamashita A, Nakanishi H, Suzuki H, Kamata R, Tanaka K, Waku K, et al. Topology of acyltransferase motifs and substrate specificity and accessibility in 1-acyl-sn-glycero-3-phosphate acyltransferase 1. Biochim et Biophys Acta. 2007;1771:1202–15.
Shindou H, Koso H, Sasaki J, Nakanishi H, Sagara H, Nakagawa KM, et al. Docosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cells. J Biol Chem. 2017;292:12054–64.
Iizuka-Hishikawa Y, Hishikawa D, Sasaki J, Takubo K, Goto M, Nagata K, et al. Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J Biol Chem. 2017;292:12065–76.
Seelow D, Schuelke M, Hildebrandt F, Nürnberg P. HomozygosityMapper-an interactive approach to homozygosity mapping. Nucleic Acids Res. 2009;37:W593–9. Web Server issue
Ansar M, Santos-Cortez RL, Saqib MA, Zulfiqar F, Lee K, Ashraf NM, et al. Mutation of ATF6 causes autosomal recessive achromatopsia. Hum Genet. 2015;134:941–50.
Proietti Onori M, Koopal B, Everman DB, Worthington JD, Jones JR, Ploeg MA, et al. The intellectual disability-associated CAMK2G p.Arg292Pro mutation acts as a pathogenic gain-of-function. Hum Mutat. 2018;39:2008–24.
Küry S, van Woerden GM, Besnard T, Proietti Onori M, Latypova X, Towne MC, et al. De novo mutations in protein kinase genes CAMK2A and CAMK2B cause intellectual disability. Am J Hum Genet. 2017;101:768–88.
Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–72.
Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300). Eur J Hum Genet. 2007;15:511–21.
Hentze MW, Kulozik AE. A perfect message: RNA surveillance and nonsense-mediated decay. Cell. 1999;96:307–10.
Kooijman EE, Chupin V, de Kruijff B, Burger KN. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic (Cph, Den). 2003;4:162–74.
Acknowledgements
We acknowledge the volunteer participation of patients and other members of the family. We are thankful to the Higher Education Commission of Pakistan for providing International Research Support Fellowship to Madiha Amin Malik. Deborah A Nickerson made significant contributions to the work. Dr. Nickerson was a very distinguished and highly respected researcher who passed away too early. Therefore, we acknowledge her work and the impact that she and her research activities are still making. We are also thankful to University of Washington Center for Mendelian Genomics for their support in the exome sequencing.
Funding
The research was supported by National Research Grant (NRPU-7099) to Muhammad Ansar by Higher Education Commission of Pakistan, International Research Support Fellowship (IRSIP) to Madiha Amin Malik by Higher Education Commission of Pakistan and by the National Institute of Health – National Institute of Child Health and Development grant R01 HD109342 to Suzanne M. Leal.
Author information
Authors and Affiliations
Contributions
Conceptualization and study design: MAM, YE, SML, MA; Family recruitment and clinical tests: MANS; Homozygosity mapping (HB) and exome sequencing (ES): AA, IS, MJB, RLPS-C; HB and ES data analysis: MAM, MANS, AA, RLPS-C; AGPAT3 constructs and western blotting (WB) experiments and data analysis: MAM, EM; In utero electroporation (IUE) experiments: MAM, IW; IUE data analysis: MAM; First draft of manuscript: MAM, IS, SML, MA: Manuscript review and editing: MAM, EM, MRA, YE, SML, MA. All co-authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
It is certified that the study was performed in accordance with the ethical standards of 1964 Helsinki Declaration. The study was conducted by obtaining ethical approval from Bio-Ethics Committee (BEC-562) of Faculty of Biological Sciences, Quaid-I-Azam University, Islamabad, Pakistan and Institutional Review Board (IRB), at Columbia University (IRB-AAAS3433), USA. Animal experiments were performed in accordance with European Commission Council Directive 2010/63/EU (CCD approval AVD101002017893) at Erasmus Medical Center, Rotterdam, Netherlands.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malik, M.A., Saqib, M.A.N., Mientjes, E. et al. A loss of function variant in AGPAT3 underlies intellectual disability and retinitis pigmentosa (IDRP) syndrome. Eur J Hum Genet 31, 1447–1454 (2023). https://doi.org/10.1038/s41431-023-01475-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01475-w
This article is cited by
-
2023 in the European Journal of Human Genetics
European Journal of Human Genetics (2024)
-
Ambivalence and regret in genome sequencing
European Journal of Human Genetics (2023)