Abstract
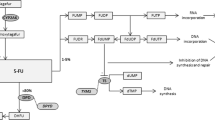
The Dutch Pharmacogenetics Working Group (DPWG) aims to facilitate PGx implementation by developing evidence-based pharmacogenetics guidelines to optimize pharmacotherapy. This guideline describes the gene-drug interaction of ABCG2 with allopurinol, HLA-B with allopurinol, MTHFR with folic acid, and MTHFR with methotrexate, relevant for the treatment of gout, cancer, and rheumatoid arthritis. A systematic review was performed based on which pharmacotherapeutic recommendations were developed. Allopurinol is less effective in patients with the ABCG2 p.(Gln141Lys) variant. In HLA-B*58:01 carriers, the risk of severe cutaneous adverse events associated with allopurinol is strongly increased. The DPWG recommends using a higher allopurinol dose in patients with the ABCG2 p.(Gln141Lys) variant. For HLA-B*58:01 positive patients the DPWG recommends choosing an alternative (for instance febuxostat). The DPWG indicates that another option would be to precede treatment with allopurinol tolerance induction. Genotyping of ABCG2 in patients starting on allopurinol was judged to be ‘potentially beneficial’ for drug effectiveness, meaning genotyping can be considered on an individual patient basis. Genotyping for HLA-B*58:01 in patients starting on allopurinol was judged to be ‘beneficial’ for drug safety, meaning it is advised to consider genotyping the patient before (or directly after) drug therapy has been initiated. For MTHFR-folic acid there is evidence for a gene-drug interaction, but there is insufficient evidence for a clinical effect that makes therapy adjustment useful. Finally, for MTHFR-methotrexate there is insufficient evidence for a gene-drug interaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data and material are either included in the supplementary information or publicly available (i.e., the published articles, PubMed). The guidelines and background information are available on KNMP.nl [1] and will be available on PharmGKB.org.
References
Royal Dutch Pharmacists Association (KNMP): Pharmacogenetic Recommendation Text [cited 2022 2nd of January]. Available from: https://www.knmp.nl/.
(EACPT) EAfCPaT. [cited 2022 2nd of January]. Available from: https://www.eacpt.eu/.
(EAHP) EAoHP. [cited 2022 2nd of January]. Available from: https://www.eahp.eu/.
Amstutz U, Henricks LM, Offer SM, Barbarino J, Schellens JHM, Swen JJ, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 Update. Clin Pharm Ther. 2018;103:210–6.
Bank PCD, Caudle KE, Swen JJ, Gammal RS, Whirl-Carrillo M, Klein TE, et al. Comparison of the guidelines of the clinical pharmacogenetics implementation consortium and the Dutch pharmacogenetics working group. Clin Pharm Ther. 2018;103:599–618.
Lunenburg CATC, van der Wouden CH, Nijenhuis M, Crommentuijn-van Rhenen MH, de Boer-Veger NJ, Buunk AM, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction of DPYD and fluoropyrimidines. Eur J Hum Genet. 2020;28:508–17.
Swen J, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee A, Mulder H, et al. Pharmacogenetics: From Bench to Byte— An Update of Guidelines. Clin Pharmacol Therapeutics. 2011;89:662–73.
Swen J, Wilting I, de Goede A, Grandia L, Mulder H, Touw D, et al. Pharmacogenetics: From Bench to Byte. Clin Pharmacol Therapeutics. 2008;83:781–7.
Matic M, Nijenhuis M, Soree B, de Boer-Veger NJ, Buunk A-M, Houwink EJF, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction between CYP2D6 and opioids (codeine, tramadol and oxycodone). Eur J Human Genet. 2021. https://doi.org/10.1038/s41431-021-00920-y. Epub ahead of print.
Brouwer J, Nijenhuis M, Soree B, Guchelaar HJ, Swen JJ, van Schaik RHN, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur J Hum Genet. 2021. https://doi.org/10.1038/s41431-021-01004-7. Epub ahead of print.
Lin CW, Huang WI, Chao PH, Chen WW, Hsiao FY. Risk of cutaneous adverse reactions associated with allopurinol or febuxostat in real-world patients: A nationwide study. Int J Clin Pr. 2019;73:e13316.
Jung JW, Kim DK, Park HW, Oh KH, Joo KW, Kim YS, et al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet Med. 2015;17:807–14.
Saksit N, Tassaneeyakul W, Nakkam N, Konyoung P, Khunarkornsiri U, Chumworathayi P, et al. Risk factors of allopurinol-induced severe cutaneous adverse reactions in a Thai population. Pharmacogenet Genomics. 2017;27:255–63.
Keller SF, Lu N, Blumenthal KG, Rai SK, Yokose C, Choi JWJ, et al. Racial/ethnic variation and risk factors for allopurinol-associated severe cutaneous adverse reactions: a cohort study. Ann Rheum Dis. 2018;77:1187–93.
Inoue K, Yuasa H. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab Pharmacokinet. 2014;29:12–9.
Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. EurHeart J. 2006;27:2400–5.
NCBI gene database [Internet]. NCBI: National Center for Biotechnology Information. 2021 [cited 11/08/2021]. Available from: https://www.ncbi.nlm.nih.gov/gene/.
Eckenstaler R, Benndorf RA. The Role of ABCG2 in the Pathogenesis of Primary Hyperuricemia and Gout-An Update. Int J Mol Sci. 2021;22:6678.
Nakamura M, Fujita K, Toyoda Y, Takada T, Hasegawa H, Ichida K. Investigation of the transport of xanthine dehydrogenase inhibitors by the urate transporter ABCG2. Drug Metab Pharmacokinet. 2018;33:77–81.
Chen L, Manautou JE, Rasmussen TP, Zhong XB. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm Sin B 2019;9:659–74.
Li R, Miao L, Qin L, Xiang Y, Zhang X, Peng H, et al. A meta-analysis of the associations between the Q141K and Q126X ABCG2 gene variants and gout risk. Int J Clin Exp Pathol. 2015;8:9812–23.
Nucleotide [Internet]: Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [Available from: https://www.ncbi.nlm.nih.gov/nuccore/EU499350.1.
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3.
van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62:1044–51.
Du B, Tian H, Tian D, Zhang C, Wang W, Wang L, et al. Genetic polymorphisms of key enzymes in folate metabolism affect the efficacy of folate therapy in patients with hyperhomocysteinaemia. Br J Nutr. 2018;119:887–95.
Soukup T, Dosedel M, Pavek P, Nekvindova J, Barvik I, Bubancova I, et al. The impact of C677T and A1298C MTHFR polymorphisms on methotrexate therapeutic response in East Bohemian region rheumatoid arthritis patients. Rheumatol Int. 2015;35:1149–61.
Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;2013:Cd000951.
Swen JJ, Nijenhuis M, van Rhenen M, de Boer-Veger NJ, Buunk AM, Houwink EJF, et al. Pharmacogenetic Information in Clinical Guidelines: The European Perspective. Clin Pharm Ther. 2018;103:795–801.
Saito Y, Stamp LK, Caudle KE, Hershfield MS, McDonagh EM, Callaghan JT, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin Pharm Ther. 2016;99:36–7.
Pharmacovigilance Working Party (PhVWP) monthly report on safety concerns, guidelines and general matters - July 2012: Allopurinol: risk of skin reactions associated with HLA-B*5801 allele: European Medicine Agency CFMPFHU, Pharmacovigilance Working Party; 2012 [Available from: https://www.ema.europa.eu/en/monthly-reports-chmp-pharmacovigilance-working-party.
Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431–46.
Acknowledgements
We dedicate this article to Bob Wilffert, who sadly passed away last year. He was a much respected member of our pharmacogenetic working group for many years.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 668353. The DPWG received funding from the Royal Dutch Pharmacists Association. G.A. Rongen received funding from the ZonMw programme ‘Goed Gebruik Geneesmiddelen’: grant number 848044004 ‘AllopuriNol to treat AtheRosClerosis in patients with Hyperuricemia (ANARCHY)’.
Author information
Authors and Affiliations
Contributions
KP drafted the manuscript and contributed to interpretation of results. EH and GR supervised drafting of the manuscript and contributed to conceiving the work and interpretation of the results. MN contributed to conceiving the work and interpretation of the results, and performed the data extraction. BS drafted and published English versions of clinical decision support texts. NBV, AB, HG, AR, RS, JS, DT, JW, RW, and VD contributed to conceiving the work and interpretation of the results. In addition, all authors revised the manuscript and approved the final version as well as agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was not required as no individual patient data was used for this article. Data was extracted from other publications and cannot be traced to any individual patient.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van der Pol, K.H., Nijenhuis, M., Soree, B. et al. Dutch pharmacogenetics working group guideline for the gene-drug interaction of ABCG2, HLA-B and Allopurinol, and MTHFR, folic acid and methotrexate. Eur J Hum Genet 32, 155–162 (2024). https://doi.org/10.1038/s41431-022-01180-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01180-0