Abstract
Background & Aim
Patatin-like phospholipase domain-containing 3 gene (PNPLA3) polymorphism has been implicated in susceptibility to non-alcoholic fatty liver disease (NAFLD), with evidence for potential interaction with nutrition. However, the combination of meat consumption with genetic polymorphism has not been tested. Therefore, this study aims to test the association between the joint presence of PNPLA3 rs738409 G-allele with high meat consumption and NAFLD in populations with diverse meat consumption.
Methods
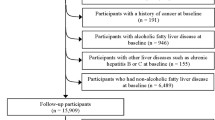
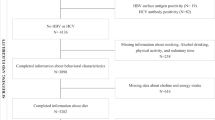
A cross-sectional study among Israeli screening and Brazilian primary healthcare populations. Food consumption was assessed by a food-frequency questionnaire. PNPLA3 polymorphism was defined as homozygous (GG) or heterozygous (GC). Inconclusive/probable NAFLD was defined as a fatty liver index (FLI) ≥ 30 and probable NAFLD as FLI ≥ 60.
Results
The sample included 511 subjects from the screening and primary healthcare populations (n = 213 and n = 298, respectively). Genetic polymorphism (homozygous GG or heterozygous GC) combined with high consumption of total meat, red and/or processed meat, unprocessed red meat, and processed meat was associated with the highest odds for inconclusive/probable NAFLD (OR = 2.75, 95%CI 1.27–5.97, p = 0.011; OR = 3.24, 1.43–7.34, p = 0.005; OR = 2.92, 1.32–6.47, p = 0.008; OR = 3.16, 1.46–6.83, p = 0.003, respectively), adjusting for age, gender, BMI, alcohol consumption, carbohydrate, and saturated fat intake. In addition, genetic polymorphism combined with high processed meat consumption was associated with the highest odds for probable NAFLD (OR = 2.40, 95%CI 1.04–5.56, p = 0.040).
Conclusions
High red meat intake may confer a greater risk for NAFLD among PNPLA3 polymorphism carriers. Prospective studies are needed to confirm these findings and consider minimizing red and processed meat consumption among PNPLA3 polymorphism carriers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to ethical considerations, but are available from the corresponding author on reasonable request.
References
Henry L, Paik J, Younossi ZM. Review article: the epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment Pharmacol Ther. 2022;56:942–56.
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61.
Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Journal of Gastroenterology and Hepatology. 2021;36:1470–8.
Kawaguchi T, Charlton M, Kawaguchi A, Yamamura S, Nakano D, Tsutsumi T, et al. Effects of Mediterranean diet in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression analysis of randomized controlled trials. Seminars in Liver Disease; 2021: Thieme Medical Publishers, Inc.; 2021. p. 225-34.
Ivancovsky-Wajcman D, Fliss-Isakov N, Webb M, Bentov I, Shibolet O, Kariv R, et al. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int 2021.
He K, Li Y, Guo X, Zhong L, Tang S. Food groups and the likelihood of non-alcoholic fatty liver disease: a systematic review and meta-analysis. British Journal of Nutrition. 2020;124:1–13.
Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss Isakov N, Webb M, Orenstein D, Shibolet O, et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68:1239–46.
Noureddin M, Zelber‐Sagi S, Wilkens LR, Porcel J, Boushey CJ, Le Marchand L, et al. Diet associations with nonalcoholic fatty liver disease in an ethnically diverse population: the Multiethnic Cohort. Hepatology. 2020;71:1940–52.
Soleimani D, Ranjbar G, Rezvani R, Goshayeshi L, Razmpour F, Nematy M. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2019;12:315–24.
Lahelma M, Luukkonen PK, Qadri S, Ahlholm N, Lallukka-Brück S, Porthan K, et al. Assessment of lifestyle factors helps to identify liver fibrosis due to non-alcoholic fatty liver disease in obesity. Nutrients. 2021;13:169.
Hashemian M, Merat S, Poustchi H, Jafari E, Radmard A-R, Kamangar F, et al. Red meat consumption and risk of nonalcoholic fatty liver disease in a population with low meat consumption: the golestan cohort study. Am J Gastroenterol. 2021;116:1667–75.
Kim MN, Lo C-H, Corey KE, Luo X, Long L, Zhang X, et al. Red meat consumption, obesity, and the risk of nonalcoholic fatty liver disease among women: evidence from mediation analysis. Clin Nutr. 2022;41:356–64.
Ivancovsky-Wajcman D, Fliss-Isakov N, Grinshpan LS, Salomone F, Lazarus JV, Webb M, et al. High meat consumption is prospectively associated with the risk of non-alcoholic fatty liver disease and presumed significant fibrosis. Nutrients. 2022;14:3533.
Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247–55.
Wang X, Liu Z, Wang K, Wang Z, Sun X, Zhong L, et al. Additive effects of the risk alleles of PNPLA3 and TM6SF2 on non-alcoholic fatty liver disease (NAFLD) in a Chinese population. Front Genet. 2016;7:140.
Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5.
Sookoian S, Pirola CJ. Meta‐analysis of the influence of I148M variant of patatin‐like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–94.
Davis JN, Le KA, Walker RW, Vikman S, Spruijt-Metz D, Weigensberg MJ, et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr. 2010;92:1522–7.
Santoro N, Savoye M, Kim G, Marotto K, Shaw MM, Pierpont B, et al. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS One. 2012;7:e37827.
Vilar-Gomez E, Pirola CJ, Sookoian S, Wilson LA, Belt P, Liang T, et al. Impact of the Association Between PNPLA3 Genetic Variation and Dietary Intake on the Risk of Significant Fibrosis in Patients With NAFLD. Am J Gastroenterol. 2021;116:994–1006.
Souza Ade M, Pereira RA, Yokoo EM, Levy RB, Sichieri R. Most consumed foods in Brazil: National Dietary Survey 2008-2009. Rev Saude Publica. 2013;47:190S–199S.
OECD-FAO Agricultural Outlook (Edition 2021) https://data.oecd.org/agroutput/meat-consumption.htm.
Santana NMT, Mill JG, Velasquez-Melendez G, Moreira AD, Barreto SM, Viana MC, et al. Consumption of alcohol and blood pressure: Results of the ELSA-Brasil study. PLoS One. 2018;13:e0190239.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23.
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Wilett W. Nutritional epidemiology: New York: Oxford University Press; 1998.
Cantwell M, Mittl B, Curtin J, Carroll R, Potischman N, Caporaso N, et al. Relative validity of a food frequency questionnaire with a meat-cooking and heterocyclic amine module. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2004;13:293–8.
Keinan-Boker L, Noyman N, Chinich A, Green MS, Nitzan-Kaluski D. Overweight and obesity prevalence in Israel: findings of the first national health and nutrition survey (MABAT). Isr Med Assoc J. 2005;7:219–23.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:1–7.
Tabela brasileira de composição de alimentos: Núcleo de Estudos e Pesquisas em Alimentação. Universidade Estadual de Campinas; 2011.
Dehmer GJ, Badhwar V, Bermudez EA, Cleveland JC Jr, Cohen MG, D’Agostino RS, et al. 2020 AHA/ACC key data elements and definitions for coronary revascularization: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop clinical data standards for coronary revascularization). J Am College Cardiol. 2020;75:1975–2088.
Dai G, Liu P, Li X, Zhou X, He S. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease (NAFLD) susceptibility and severity: A meta-analysis. Medicine (Baltimore). 2019;98:e14324.
Yoshimura SM, Duarte SMB, Stefano JT, Mazo DFC, Pinho JRR, Oliveira CP. Pnpla3 gene polymorphism and red meat consumption increased fibrosis risk in nash biopsy-proven patients under medical follow-up in a tertiary center in Southwest Brazil. Arq Gastroenterol. 2023;60:98–105.
Peng H, Xie X, Pan X, Zheng J, Zeng Y, Cai X, et al. Association of meat consumption with NAFLD risk and liver-related biochemical indexes in older Chinese: a cross-sectional study. BMC Gastroenterol. 2021;21:221.
Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue-Choi M, Dawsey SM, et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957.
He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–15.
Bordoni L, Petracci I, Zhao F, Min W, Pierella E, Assmann TS, et al. Nutrigenomics of dietary lipids. Antioxidants (Basel). 2021;10:994.
Castellana M, Donghia R, Guerra V, Procino F, Lampignano L, Castellana F, et al. Performance of fatty liver index in identifying non-alcoholic fatty liver disease in population studies. A meta-analysis. J Clin Med. 2021;10:1877.
Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171:561–9.
Kim KS, Hong S, Han K, Park CY. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study. BMJ. 2024;384:e076388.
Chung GE, Jeong SM, Cho EJ, Yoon JW, Yoo JJ, Cho Y, et al. The association of fatty liver index and BARD score with all-cause and cause-specific mortality in patients with type 2 diabetes mellitus: a nationwide population-based study. Cardiovasc Diabetol. 2022;21:273.
Siafi E, Andrikou I, Thomopoulos C, Konstantinidis D, Kakouri N, Tatakis F, et al. Fatty liver index and cardiovascular outcomes in never-treated hypertensive patients: a prospective cohort. Hypertens Res. 2023;46:119–27.
Cuthbertson DJ, Koskinen J, Brown E, Magnussen CG, Hutri-Kahonen N, Sabin M, et al. Fatty liver index predicts incident risk of prediabetes, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Ann Med. 2021;53:1256–64.
Funding
This study was financed in part by: Research Grants and Fellowships Fund on Food and Nutrition and their Implications on Public Health, The Israeli Ministry of Health. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) -Finance Code 001. Conselho Nacional de DesenvolvimentoCientífico e Tecnológico, Brazil.
Author information
Authors and Affiliations
Contributions
MRAS: study concept and design, analysis and interpretation of data, drafting of the manuscript; DIW: study concept and design, analysis and interpretation of data, statistical analysis; CPO: study concept and design, analysis and interpretation of data, drafting of the manuscript; SR: data collection; LL: data collection, analysis and interpretation of data, PNPLA3 polymorphism analysis; CUC: PNPLA3 polymorphism analysis; SMY: data collection; DJ: study concept and design; MBY: PNPLA3 polymorphism analysis; LSG: analysis and interpretation of data, critical revision of the manuscript for important intellectual content; OS: critical revision of the manuscript for important intellectual content; RR: study concept and design, critical revision of the manuscript for important intellectual content, study supervision; SZS: study concept and design, analysis and interpretation of data, drafting of the manuscript, study supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was aproved by the IRB of each center.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alvares-da-Silva, M.R., Ivancovsky-Wajcman, D., Oliveira, C.P. et al. High red meat consumption among PNPLA3 polymorphism carriers is associated with NAFLD in a multi-center cross-sectional study. Eur J Clin Nutr (2024). https://doi.org/10.1038/s41430-024-01416-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41430-024-01416-w