Abstract
Background/Objectives
The association between dietary acid load (DAL) and chronic kidney disease (CKD) progression remains controversial. Also, there is a gap in the literature on the association between DAL and mortality. In this study, we evaluated the association between NEAP (net endogenous acid production) and PRAL (potential renal acid load) and the risk of events of all-cause mortality and kidney replacement therapy (KRT) in people with CKD.
Subjects/Methods
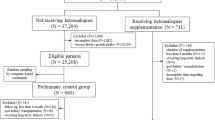
We included 442 patients (250 diabetics) from the Progredir Cohort Study, based in São Paulo, Brazil. We estimated NEAP and PRAL from dietary intake. Events of death before KRT and KRT were ascertained after a median follow-up of 5.8 and 5.1 years, respectively. Cox proportional hazards regression, Weibull regression, and competing risk models were performed.
Results
Median NEAP and PRAL were 49.5 and 4.8 mEq/d. There were 200 deaths and 75 KRT events. Neither NEAP nor PRAL were associated with mortality or KRT when all participants were analyzed. After stratification for diabetes, both estimates were positively related to the risk of KRT even after adjustment for age, sex, weight status, glomerular filtration rate, serum bicarbonate, and intakes of protein, phosphorus, and energy (HR 1.31; 95% CI 1.07, 1.60 for NEAP, and HR 1.27; 95% CI 1.04, 1.57 for every 10 mEq/d increments). Competing risk analyses confirmed these findings.
Conclusions
DAL estimates were associated with the risk of KRT in people with CKD and diabetes but not in non-diabetics. There was no association between all-cause mortality and DAL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending.
References
Frassetto LA, Todd KM, Morris RC Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68:576–83.
Passey C. Reducing the dietary acid load: how a more alkaline diet benefits patients with chronic kidney disease. J Ren Nutr. 2017;27:151–60.
Ströhle A, Hahn A, Sebastian A. Estimation of the diet-dependent net acid load in 229 worldwide historically studied hunter-gatherer societies. Am J Clin Nutr. 2010;91:406–12.
Arisawa K, Katsuura-Kamano S, Uemura H, Tien NV, Hishida A, Tamura T, et al. Association of dietary acid load with the prevalence of metabolic syndrome among participants in baseline survey of the Japan Multi-Institutional Collaborative Cohort study. Nutrients. 2020;12:1605.
Sanz JM, Sergi D, Colombari S, Capatti E, Situlin R, Biolo G, et al. Dietary acid load but not Mediterranean diet adherence score is associated with metabolic and cardiovascular health state: a population observational study from Northern Italy. Front Nutr. 2022;9:828587.
Ronco AL, Martínez-López W, Calderón JM, Golomar W. Dietary acid load and lung cancer risk: a case-control study in men. Cancer Treat Res Commun. 2021;28:100382.
Shi LW, Wu YL, Hu JJ, Yang PF, Sun WP, Gao J, et al. Dietary acid load and the risk of pancreatic cancer: a prospective cohort study. Cancer Epidemiol Biomark Prev. 2021;30:1009–19.
Akter S, Nanri A, Mizoue T, Noda M, Sawada N, Sasazuki S, et al. Dietary acid load and mortality among Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2017;106:146–54.
Mazidi M, Mikhailidis DP, Banach M. Higher dietary acid load is associated with higher likelihood of peripheral arterial disease among American adults. J Diabetes Complic. 2018;32:565–9.
Hejazi E, Emamat H, Sharafkhah M, Saidpour A, Poustchi H, Sepanlou S, et al. Dietary acid load and mortality from all causes, CVD and cancer: results from the Golestan Cohort Study. Br J Nutr. 2022;128:237–43.
Rebholz CM, Coresh J, Grams ME, Steffen LM, Anderson CA, Appel LJ, et al. Dietary acid load and incident chronic kidney disease: results from the ARIC Study. Am J Nephrol. 2015;42:427–35.
Scialla JJ, Appel LJ, Astor BC, Miller ER III, Beddhu S, Woodward M, et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012;82:106–12.
Kanda E, Ai M, Kuriyama R, Yoshida M, Shiigai T. Dietary acid intake and kidney disease progression in the elderly. Am J Nephrol. 2014;39:145–52.
Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Ríos-Burrows N, et al. High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol. 2015;26:1693–1700.
Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59:1356–61.
Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76:S1–S107.
Lobene AJ, Stremke ER, McCabe GP, Moe SM, Moorthi RN, Gallant KMH. Spot urine samples to estimate Na and K intake in patients with chronic kidney disease and healthy adults: a secondary analysis from a controlled feeding study. J Ren Nutr. 2021;31:602–10.
Pike M, Stewart TG, Morse J, Ormsby P, Siew ED, Hung A, et al. APOL1, acid load, and CKD progression. Kidney Int Rep. 2019;4:946–54.
Scialla JJ, Asplin J, Dobre M, Chang AR, Lash J, Hsu CY, et al. Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int. 2017;91:204–15.
Domingos MAM, Goulart AC, Lotufo PA, Benseñor IJM, Titan SMO. Chronic kidney disease - determinants of progression and cardiovascular risk. PROGREDIR cohort study: design and methods. Sao Paulo Med J. 2017;135:133–9.
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–49.
World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: WHO; 1997.
Organización Panamericana de la Salud. Encuesta Multicéntrica Salud Bienestar y Envejecimiento (SABE) en América Latina y el Caribe: informe preliminar. Washington: OPAS; 2001.
Molina MCB, Benseñor IM, Cardoso LO, Velasquez-Melendez G, Drehmer M, Pereira TSS, et al. Reprodutibilidade e validade relativa do Questionário de Frequência Alimentar do ELSA-Brasil (Reproducibility and relative validity of the Food Frequency Questionnaire used in the ELSA-Brasil). Cad Saúde Pública. 2013;29:379–89.
United States Department of Agriculture. Agricultural Research Service. USDA Food Composition Databases. 2016. https://ndb.nal.usda.gov/ndb/.
Núcleo de Estudos e Pesquisas em Alimentação, Universidade Estadual de Campinas. Tabela Brasileira de Composição de Alimentos. 4th ed. NEPA-UNICAMP, Campinas, 2011.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S.
Willett WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998.
Machado AD, Anjos FSN, Domingos MAM, Molina MCB, Marchioni DML, Benseñor IJM, et al. Dietary intake of non-dialysis chronic kidney disease patients: the PROGREDIR study. A cross-sectional study. Sao Paulo Med J. 2018;136:208–15.
Nerbass FB, Lima HN, Thomé FS, Vieira Neto OM, Lugon JR, Sesso R. Brazilian dialysis survey 2020. J Bras Nefrol. 2022;44:349–57.
Carroll KJ. On the use and utility of the Weibull model in the analysis of survival data. Control Clin Trials. 2003;24:682–701.
Elsayed EA. Accelerated life testing. In: Pham H (ed). Handbook of reliability engineering. New York: Springer; 2003, 415–28.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–94.
Angeloco LRN, Arces de Souza GC, Romão EA, Frassetto L, Chiarello PG. Association of dietary acid load with serum bicarbonate in chronic kidney disease (CKD) patients. Eur J Clin Nutr. 2020;74:69–75.
Williams RS, Heilbronn LK, Chen DL, Coster ACF, Greenfield JR, Samocha-Bonet D. Dietary acid load, metabolic acidosis and insulin resistance - Lessons from cross-sectional and overfeeding studies in hum;ans. Clin Nutr. 2016;35:1084–90.
Juszczak F, Caron N, Mathew AV, Declèves AE. Critical role for AMPK in metabolic disease-induced chronic kidney disease. Int J Mol Sci. 2020;21:7994.
Xu H, Jia T, Huang X, Risérus U, Cederholm T, Ärnlöv J, et al. Dietary acid load, insulin sensitivity and risk of type 2 diabetes in community-dwelling older men. Diabetologia. 2014;57:1561–8.
Akter S, Eguchi M, Kuwahara K, Kochi T, Ito R, Kurotani K, et al. High dietary acid load is associated with insulin resistance: the Furukawa Nutrition and Health Study. Clin Nutr. 2016;35:453–9.
Ikizler HO, Zelnick L, Ruzinski J, Curtin L, Utzschneider KM, Kestenbaum B, et al. Dietary acid load is associated with serum bicarbonate but not insulin sensitivity in chronic kidney disease. J Ren Nutr. 2016;26:93–102.
Gæde J, Nielsen T, Madsen ML, Toft U, Jørgensen T, Overvad K, et al. Population-based studies of relationships between dietary acidity load, insulin resistance and incident diabetes in Danes. Nutr J. 2018;17:91.
Abshirini M, Bagheri F, Mahaki B, Siassi F, Koohdani F, Safabakhsh M, et al. The dietary acid load is higher in subjects with prediabetes who are at greater risk of diabetes: a case-control study. Diabetol Metab Syndr. 2019;11:52.
Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–8.
Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5:1277–81.
Sakhaee K. Recent advances in the pathophysiology of nephrolithiasis. Kidney Int. 2009;75:585–95.
Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76:667–75.
Wesson DE, Jo CH, Simoni J. Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int. 2012;82:1184–94.
Wesson DE, Buysse JM, Bushinsky DA. Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J Am Soc Nephrol. 2020;31:469–82.
Madias NE. Eubicarbonatemic hydrogen ion retention and CKD progression. Kidney Med. 2021;3:596–606.
Brito-Ashurst I, Varagunam M, Raftery MJ, Yagoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–84.
Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78:303–9.
Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77:617–23.
Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8:371–81.
Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol. 2019;32:989–1001.
Alva S, Divyashree M, Kamath J, Prakash PS, Prakash KS. A study on effect of bicarbonate supplementation on the progression of chronic kidney disease. Indian J Nephrol. 2020;30:91–97.
Giebisch G, Windhager E. Transport of acids and bases. In: Boron WF, Boulpaep EL (eds). Medical physiology: a cellular and molecular approach. 2nd ed. Philadelphia: Saunders Elsevier; 2009, 851–65.
Imenez Silva PH, Mohebbi N. Kidney metabolism and acid–base control: back to the basics. Pflug Arch. 2022;474:919–34.
Funding
The study received funding from the Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) (procedural no. 11/17341-0). ADM received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (procedural no. 88882.327915/2019-01). The Progredir Cohort Study used the structure of the research center for the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), in São Paulo, which was supported by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science and Technology (Financiadora de Estudos e Projetos, FINEP; and Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq) (procedural no. 01 06 0115.00 SP).
Author information
Authors and Affiliations
Contributions
ADM, PAL, IMB, and SMT designed and conducted research; ADM, DMM, and SMT provided data curation; PAL and IMB provided essential materials; ADM performed statistical analysis; ADM and SMT wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Progredir Cohort Study was approved by two Brazilian Ethics Committees (protocols 11147/11 and 0798/11), and all participants provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Machado, A.D., Marchioni, D.M., Lotufo, P.A. et al. Dietary acid load and the risk of events of mortality and kidney replacement therapy in people with chronic kidney disease: the Progredir Cohort Study. Eur J Clin Nutr 78, 128–134 (2024). https://doi.org/10.1038/s41430-023-01361-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01361-0