Abstract
Background
Previous observational studies focused on the association of coffee consumption and neurological disease. However, it is not known whether these associations are causal.
Methods
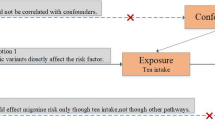
We used Mendelian randomization (MR) study to assess the causal relationship of coffee intake with the risk of neurological diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, epilepsy, stroke, and migraine. Single-nucleotide polymorphisms (SNPs) which had genetic statistical significance with coffee intake were used as instrumental variable (IV). Genetic instruments were stretched from the MRC-IEU (MRC Integrative Epidemiology Unit) analysis on the UK Biobank. We performed MR analyses using the inverse variance weighted (IVW) method as the main approach. Sensitivity analyses were further performed using MR-Egger and MR-PRESSO to assess the robustness.
Results
In the MR analysis, 40 SNPs were selected as IV, the F statistics for all SNPs ranged from 16 to 359. In IVW approach, our results provide genetic evidence supporting a potential causal association between coffee intake and a lower risk of migraine (OR = 0.528, 95% CI = 0.342–0.817, P = 0.004) and migraine with aura (OR = 0.374, 95% CI = 0.208–0.672, P = 0.001). However, we found no significant association between coffee intake and other neurological diseases along with their subtypes in this MR study.
Conclusion
Using genetic data, our MR study found significant evidence supporting a causal association between coffee intake and migraine. This suggests that coffee consumption is likely a trigger or a prevention strategy for migraine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its additional files.
References
Nehlig A. Effects of coffee/caffeine on brain health and disease: what should I tell my patients? Pract Neurol. 2016;16:89–95. https://doi.org/10.1136/practneurol-2015-001162.
Herden L, Weissert R. The impact of coffee and caffeine on multiple sclerosis compared to other neurodegenerative diseases. Front Nutr. 2018;5:133 https://doi.org/10.3389/fnut.2018.00133.
Shao C, Tang H, Wang X, He J. Coffee consumption and stroke risk: evidence from a systematic review and meta-analysis of more than 2.4 million men and women. J Stroke Cerebrovasc Dis. 2021;30:105452. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105452.
Baratloo A, Mirbaha S, Delavar Kasmaei H, Payandemehr P, Elmaraezy A, Negida A. Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J Pain. 2017;30:176–82. https://doi.org/10.3344/kjp.2017.30.3.176.
Chan L, Hong CT, Bai CH. Coffee consumption and the risk of cerebrovascular disease: a meta-analysis of prospective cohort studies. BMC Neurol. 2021;21:380. https://doi.org/10.1186/s12883-021-02411-5.
Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–904. https://doi.org/10.1056/NEJMoa1112010.
Barrea L, Pugliese G, Frias-Toral E, El Ghoch M, Castellucci B, Chapela SP, et al. Coffee consumption, health benefits and side effects: a narrative review and update for dietitians and nutritionists. Crit Rev Food Sci Nutr. 2023;63:1238–61. https://doi.org/10.1080/10408398.2021.1963207.
Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–59. https://doi.org/10.1161/CIRCULATIONAHA.113.005925.
Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 2020;19:255–65. https://doi.org/10.1016/S1474-4422(19)30411-9.
Group GBDNDC. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–97. https://doi.org/10.1016/S1474-4422(17)30299-5.
Collaborators GBDN. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80. https://doi.org/10.1016/S1474-4422(18)30499-X.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63. https://doi.org/10.1002/sim.3034.
Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36:465–78. https://doi.org/10.1007/s10654-021-00757-1.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925–6. https://doi.org/10.1001/jama.2017.17219.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779 https://doi.org/10.1371/journal.pmed.1001779.
Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30. https://doi.org/10.1038/s41588-019-0358-2.
Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–102. https://doi.org/10.1016/S1474-4422(19)30320-5.
Nicolas A, Kenna KP, Renton AE, Ticozzi N, Faghri F, Chia R, et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron. 2018;97(6):1268–83.e6. https://doi.org/10.1016/j.neuron.2018.02.027.
International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365. https://doi.org/10.1126/science.aav7188
Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–37. https://doi.org/10.1038/s41588-018-0058-3. e-pub ahead of print 2018/03/14
International League Against Epilepsy Consortium on Complex Epilepsies. Genome-wide mega-analysis identifies 16 loci and highlights diverse biological mechanisms in the common epilepsies. Nat Commun. 2018;9:5269 https://doi.org/10.1038/s41467-018-07524-z.
Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–7. https://doi.org/10.1093/bioinformatics/btv402.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. https://doi.org/10.1038/s41588-018-0099-7.
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28:30–42. https://doi.org/10.1097/EDE.0000000000000559.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25. https://doi.org/10.1093/ije/dyv080.
Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–501. https://doi.org/10.1093/ije/dyt179.
Lee MJ, Choi HA, Choi H, Chung CS. Caffeine discontinuation improves acute migraine treatment: a prospective clinic-based study. J Headache Pain. 2016;17:71. https://doi.org/10.1186/s10194-016-0662-5.
Bigal ME, Sheftell FD, Rapoport AM, Tepper SJ, Lipton RB. Chronic daily headache: identification of factors associated with induction and transformation. Headache. 2002;42:575–81. https://doi.org/10.1046/j.1526-4610.2002.02143.x.
VanderPluym JH, Halker Singh RB, Urtecho M, Morrow AS, Nayfeh T, Torres Roldan VD, et al. Acute treatments for episodic migraine in adults: a systematic review and meta-analysis. JAMA. 2021;325:2357–69. https://doi.org/10.1001/jama.2021.7939.
Nowaczewska M, Wicinski M, Kazmierczak W. The ambiguous role of caffeine in migraine headache: from trigger to treatment. Nutrients. 2020;12. https://doi.org/10.3390/nu12082259.
Galeotti N, Ghelardini C, Grazioli I, Uslenghi C. Indomethacin, caffeine and prochlorperazine alone and combined revert hyperalgesia in in vivo models of migraine. Pharm Res. 2002;46:245–50. https://doi.org/10.1016/s1043-6618(02)00126-3.
Tai MS, Yap JF, Goh CB. Dietary trigger factors of migraine and tension-type headache in a South East Asian country. J Pain Res. 2018;11:1255–61. https://doi.org/10.2147/JPR.S158151.
Zheng H, Shi YZ, Liang JT, Lu LL, Chen M. Modifiable factors for migraine prophylaxis: a Mendelian randomization analysis. Front Pharm. 2023;14:1010996. https://doi.org/10.3389/fphar.2023.1010996.
Ikram M, Park TJ, Ali T, Kim MO. Antioxidant and neuroprotective effects of caffeine against Alzheimer’s and Parkinson’s disease: insight into the role of Nrf-2 and A2AR signaling. Antioxidants. 2020;9. https://doi.org/10.3390/antiox9090902.
Pourshahidi LK, Navarini L, Petracco M, Strain JJ. A comprehensive overview of the risks and benefits of coffee consumption. Compr Rev Food Sci Food Saf. 2016;15:671–84. https://doi.org/10.1111/1541-4337.12206.
Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011;51:363–73. https://doi.org/10.1080/10408390903586412.
Roshan MH, Tambo A, Pace NP. Potential role of caffeine in the treatment of Parkinson’s disease. Open Neurol J. 2016;10:42–58. https://doi.org/10.2174/1874205X01610010042.
Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–50. https://doi.org/10.1016/j.neulet.2007.12.034.
West AB. Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Exp Neurol. 2017;298:236–45. https://doi.org/10.1016/j.expneurol.2017.07.019.
Boia R, Ambrosio AF, Santiago AR. Therapeutic opportunities for caffeine and A2A receptor antagonists in retinal diseases. Ophthalmic Res. 2016;55:212–8. https://doi.org/10.1159/000443893.
Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats. J Agric Food Chem. 2014;62:116–22. https://doi.org/10.1021/jf401777m.
Jo MG, Ikram M, Jo MH, Yoo L, Chung KC, Nah SY, et al. Gintonin mitigates MPTP-induced loss of nigrostriatal dopaminergic neurons and accumulation of α-synuclein via the Nrf2/HO-1 pathway. Mol Neurobiol. 2019;56:39–55. https://doi.org/10.1007/s12035-018-1020-1.
Zhang Z, Wang M, Yuan S, Cai H, Zhu SG, Liu X. Genetically predicted coffee consumption and risk of Alzheimer’s disease and stroke. J Alzheimers Dis. 2021;83:1815–23. https://doi.org/10.3233/jad-210678.
Domenighetti C, Sugier PE, Sreelatha AAK, Schulte C, Grover S, Mohamed O, et al. Mendelian randomisation study of smoking, alcohol, and coffee drinking in relation to Parkinson’s disease. J Parkinsons Dis. 2022;12:267–82. https://doi.org/10.3233/jpd-212851.
Zhang Z, Wang M, Liu X. Genetically predicted coffee consumption and amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2022;23:575–9. https://doi.org/10.1080/21678421.2022.2047204.
Lu H, Wu PF, Zhang W, Xia K. Coffee consumption is not associated with risk of multiple sclerosis: a Mendelian randomization study. Mult Scler Relat Disord. 2020;44:102300 https://doi.org/10.1016/j.msard.2020.102300.
Zhang Z, Wang M, Yuan S, Liu X. Alcohol, coffee, and milk intake in relation to epilepsy risk. Nutrients. 2022;14. https://doi.org/10.3390/nu14061153.
Qian Y, Ye D, Huang H, Wu DJH, Zhuang Y, Jiang X, et al. Coffee consumption and risk of stroke: a Mendelian randomization study. Ann Neurol. 2020;87:525–32. https://doi.org/10.1002/ana.25693.
Acknowledgements
We extend our sincere thanks to Ben Elsworth for releasing GWAS summary statistics for coffee intake. The AD data was obtained from the IGAP consortium, the PD data from the IPDGC consortium, the ALS data from the AVS consortium, the MS data from the IMSGC consortium, and the epilepsy and its subtypes data from the ILAE consortium. We also thank the MEGASTROKE Consortium for providing summary statistics data for stroke and its subtypes. The data for nontraumatic intracranial hemorrhage, subarachnoid hemorrhage, and migraine were obtained from the FinnGen consortium. We sincerely thank all participants for sharing the genome-wide summary statistics.
Funding
This research was funded by the National Natural Science Foundation of China (82260225), the Natural Science Foundation of Jiangxi Province (20202BAB216043), and Jiangxi Province Postgraduate Innovation Special Fund Project (YC2022-B061).
Author information
Authors and Affiliations
Contributions
JZ, MZ, and DZ designed the research. JZ, YL, GX, XC, and WW acquired and analyzed data. JZ drafted the manuscript. MZ and DZ made critical revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Liu, Y., Xu, G. et al. Causal relationship between coffee intake and neurological diseases: a Mendelian randomization study. Eur J Clin Nutr 78, 114–119 (2024). https://doi.org/10.1038/s41430-023-01355-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01355-y