Abstract
Background
Previous research has shown the efficacy of mulberry extracts for lowering post-prandial glucose (PPG) responses. The postulated mechanism is slowing of glucose absorption, but effects on glucose disposal or endogenous production are also possible. This research assessed the effect of a specified mulberry fruit extract (MFE) on these three glucose flux parameters.
Methods
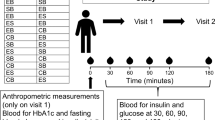
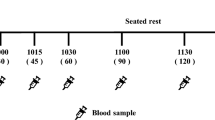
The study used a double-blind, randomized, controlled, full cross-over design. In 3 counter-balanced treatments, 12 healthy adult male subjects, mean (SD) age 24.9 (2.50) years and body mass index 22.5 (1.57) kg/m2, consumed porridge prepared from 13C-labelled wheat, with or without addition of 0.75 g MFE, or a solution of 13C-glucose in water. A co-administered 2H-glucose venous infusion allowed for assessment of glucose disposal. Glucose flux parameters, cumulative absorption (time to 50% absorption, T50%abs), and PPG positive incremental area under the curve from 0 to 120 min (+iAUC0–120) were determined from total and isotopically labelled glucose in plasma. As this exploratory study was not powered for formal inferential statistical tests, results are reported as the mean percent difference (or minutes for T50%abs) between treatments with 95% CI.
Results
MFE increased mean T50%abs by 10.2 min, (95% CI 3.9–16.5 min), and reduced mean 2 h post-meal rate of glucose appearance by 8.4% (95% CI −14.9 to −1.4%) and PPG + iAUC0-120 by 11% (95% CI −26.3 to −7.3%), with no significant changes in glucose disposal or endogenous production.
Conclusions
The PPG-lowering effect of MFE is primarily mediated by a reduced rate of glucose uptake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during the current study are not publicly available as the participants did not give express consent for this, but are available on reasonable request, noting that some caveats may apply.
References
Blaak E, Antoine JM, Benton D, Björck I, Bozzetto L, Brouns F, et al. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13:923–84.
Ceriello A, Colagiuri S. International Diabetes Federation guideline for management of postmeal glucose: a review of recommendations. Diabet Med. 2008;25:1151–6.
Livesey G, Taylor R, Livesey H, Liu S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am J Clin Nutr. 2013;97:584–96.
Thomas D, Elliott E. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;1:CD006296.
Phimarn W, Wichaiyo K, Silpsavikul K, Sungthong B, Saramunee K. A meta-analysis of efficacy of Morus alba Linn. to improve blood glucose and lipid profile. Eur J Nutr. 2017;56:1509–21.
Lown M, Fuller R, Lightowler H, Fraser A, Gallagher A, Stuart B, et al. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: results of a randomised double-blind placebo-controlled study. PLoS ONE. 2017;12:e0172239.
Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, et al. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. 2007;55:5869–74.
Mela DJ, Cao X-Z, Govindaiah S, Hiemstra H, Kalathil R, Lin L, et al. Dose-response efficacy of mulberry fruit extract for reducing post-prandial blood glucose and insulin responses: randomized trial evidence in healthy adults. Br J Nutr. 2022:1–8, https://doi.org/10.1017/S0007114522000824.
Oku T, Yamada M, Nakamura M, Sadamori N, Nakamura S. Inhibitory effects of extractives from leaves of Morus alba on human and rat small intestinal disaccharidase activity. Br J Nutr. 2006;95:933–8.
van de Laar FA, Lucassen PBLJ, Akkermans RP, van de Lisdonk EH, Rutten GEHM, van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus (Review). Cochrane Libr. 2005;2:CD003639.
Standl E, Schnell O. Alpha-glucosidase inhibitors 2012 - cardiovascular considerations and trial evaluation. Diabetes Vasc Dis Res. 2012;9:163–9.
Joshi SR, Standl E, Tong N, Shah P, Kalra S, Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother. 2015;16:1959–81.
Mela DJ, Cao X-Z, Dobriyal R, Fowler MI, Li L, Manoj J, et al. The effect of 8 plant extracts and combinations on post-prandial blood glucose and insulin responses in healthy adults: a randomized controlled trial. Nutr Metab. 2020;17:51.
Józefczuk J, Malikowska K, Glapa A, Stawińska-Witoszyńska B, Nowak JK, Bajerska J, et al. Mulberry leaf extract decreases digestion and absorption of starch in healthy subjects—a randomized, placebo-controlled, crossover study. Adv Med Sci. 2017;62:302–6.
Boers HM, Alssema M, Mela DJ, Peters HP, Vonk RJ, Priebe MG. The rate of glucose appearance is related to postprandial glucose and insulin responses in adults: a systematic review and meta-analysis of stable isotope studies. J Nutr. 2019;149:1896–903.
Eelderink C, Moerdijk-Poortvliet TCW, Wang H, Schepers M, Preston T, Boer T, et al. The glycemic response does not reflect the in vivo starch digestibility of fiber-rich wheat products in healthy men. J Nutr. 2012;142:258–63.
Eelderink C, Schepers M, Preston T, Vonk RJ, Oudhuis L, Priebe MG. Slowly and rapidly digestible starchy foods can elicit a similar glycemic response because of differential tissue glucose uptake in healthy men. Am J Clin Nutr. 2012;96:1017–24.
Nazare JA, Normand S, Oste Triantafyllou A, Brac de la Perrière A, Desage M, Laville M. Modulation of the postprandial phase by β‐glucan in overweight subjects: effects on glucose and insulin kinetics. Mol Nutr Food Res. 2009;53:361–9.
Priebe MG, Wachters-Hagedoorn RE, Heimweg JA, Small A, Preston T, Elzinga H, et al. An explorative study of in vivo digestive starch characteristics and postprandial glucose kinetics of wholemeal wheat bread. Eur J Nutr. 2008;47:417–23.
Schenk S, Davidson CJ, Zderic TW, Byerley LO, Coyle EF. Different glycemic indexes of breakfast cereals are not due to glucose entry into blood but to glucose removal by tissue. Am J Clin Nutr. 2003;78:742–8.
Boers HM, van Dijk TH, Hiemstra H, Hoogenraad AR, Mela DJ, Peters HPF, et al. Effect of fibre additions to flatbread flour mixes on glucose kinetics: a randomised controlled trial. Br J Nutr. 2017;118:777–87.
Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–91.
Williams EJ. Experimental designs balanced for the estimation of residual effects of treatments. Aust J Chem. 1949;2:149–68.
van Dijk TH, Boer TS, Havinga R, Stellaard F, Kuipers F, Reijngoud D-J. Quantification of hepatic carbohydrate metabolism in conscious mice using serial blood and urine spots. Anal Biochem. 2003;322:1–13.
Lee WNP, Bergner EA, Guo Z. Mass isotopomer pattern and precursor‐product relationship. Biol Mass Spectrom. 1992;21:114–22.
Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24.
Debodo RC, Steele R, Altszuler N, Dunn A, Bishop JS. On the hormonal regulation of carbohydrate metabolism: studies with 14C. Recent Prog Horm Res. 1963;19:445–88.
Radziuk J. Tracer methods and the metabolic disposal of a carbohydrate load in man. Diabetes Metab Rev. 1987;3:231–67.
Tissot S, Normand S, Guilluy R, Pachiaudi C, Beylot M, Laville M, et al. Use of a new gas chromatograph isotope ratio mass spectrometer to trace exogenous 13C labelled glucose at a very low level of enrichment in man. Diabetologia 1990;33:449–56.
Shreeve WW, Cerasi E, Luft R. Metabolism of [2-14C] pyruvate in normal, acromegalic and hgh-treated human subjects. Acta Endocrinol. 1970;65:155–69.
Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6.
FAO. Carbohydrates in human nutrition (FAO Food and Nutrition Paper-66). Report of a Joint FAO/WHO Expert Consultation. Food and Agriculture Organization. Rome; 1998.
Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–50.
Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M, et al. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007;21:366–77.
Livesey G, Wilson P, Dainty JR, Brown J, Faulks R, Roe M, et al. Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am J Physiol Endocrinol Metab. 1998;275:E717–28.
Elleri D, Allen J, Harris J, Kumareswaran K, Nodale M, Leelarathna L, et al. Absorption patterns of meals containing complex carbohydrates in type 1 diabetes. Diabetologia. 2013;56:1108–17.
Péronnet F, Meynier A, Sauvinet V, Normand S, Bourdon E, Mignault D, et al. Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: effect of starch digestibility. Eur J Clin Nutr. 2015;69:740–5.
Rizza RA, Toffolo G, Cobelli C. Accurate measurement of postprandial glucose turnover: why is it difficult and how can it be done (relatively) simply? Diabetes. 2016;65:1133–45.
Haidar A, Elleri D, Allen J, Harris J, Kumareswaran K, Nodale M, et al. Validity of triple-and dual-tracer techniques to estimate glucose appearance. Am J Physiol Endocrinol Metab. 2012;302:E1493–E501.
Pasmans K, Meex RC, Trommelen J, Senden JM, Vaughan EE, van Loon LJ, et al. L-arabinose co-ingestion delays glucose absorption derived from sucrose in healthy men and women: a double-blind, randomised crossover trial. Br J Nutr. 2022;128:1072–81.
Delarue J, Normand S, Pachiaudi C, Beylot M, Lamisse F, Riou J. The contribution of naturally labelled 13C fructose to glucose appearance in humans. Diabetologia. 1993;36:338–45.
Basu R, Di Camillo B, Toffolo G, Basu A, Shah P, Vella A, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol-Endocrinol Metab. 2003;284:E55–69.
Chen X, Sohouli MH, Nateghi M, Melekoglu E, Fatahi S. Impact of mulberry consumption on cardiometabolic risk factors: a systematic review and meta-analysis of randomized-controlled trials. J Clin Pharm Ther. 2022;47:1982–93.
Cui W, Luo K, Xiao Q, Sun Z, Wang Y, Cui C, et al. Effect of mulberry leaf extracts on glycemic traits: a systematic review and meta-analysis. Food Funct. 2023;14:1277–89.
Acknowledgements
Jeroen Sterken and Anton Porcu are kindly acknowledged for product handling and logistical support for samples, data and meals. Duncan Talbot is acknowledged for executing the analysis of GLP-1, GIP and glucagon.
Funding
This study was funded entirely by Unilever, and Unilever employees collaborated with the academic authors in the study hypothesis/design, execution, analysis and interpretation. The trial was carried out at an independent contract research organization. Unilever employees had no part in the study execution, participant contact or data collection, and were blind to treatment codes which were only revealed when the data were unlocked. The authors confirm that: •Industry funding was transparent, acknowledged, and appropriately recognized throughout all stages of design, implementation, and reporting. •The project design, implementation, analysis, and interpretation was performed with efforts to maximize academic independence in each of these areas. •There was full academic independence to report and publish all the findings. •The raw data can be made available to interested scientists if requested, with the understanding that there could be reasonable caveats for such requests. The test materials are commercially accessible, and the methods provide sufficient detail for independent replication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design and protocol development, data interpretation, and writing and reviewing of the manuscript. A-RH managed the interface between the sponsor and study site, and monitoring of data collection. HH was primarily responsible for the statistical design and analyses, which were agreed with all authors in advance of study recruitment. All authors have seen and approved the manuscript, and agreed that any questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved and documented.
Corresponding authors
Ethics declarations
Competing interests
HMB and A-RH are employees of Unilever, the sponsor of the study and a manufacturer of carbohydrate-containing foods. GSD, HH and DJM were employees of Unilever at the time the research was designed and conducted, but have no current affiliation with the company, and no other competing interests in the topic of this research.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and the protocol approved by the Medical Ethics Review Committee Brabant (Medische Ethische ToetsingsCommissie Brabant, Tilburg, The Netherlands) on 1 December 2015. All participants provided written informed consent. The protocol was registered at www.clinicaltrials.gov (number NCT02662738) prior to undertaking any procedures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boers, H.M., van Dijk, T.H., Duchateau, G.S. et al. Effect of mulberry fruit extract on glucose fluxes after a wheat porridge meal: a dual isotope study in healthy human subjects. Eur J Clin Nutr 77, 741–747 (2023). https://doi.org/10.1038/s41430-023-01282-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01282-y