Abstract
Background
Vitamin D is an important regulator of calcium. Mendelian randomization (MR) studies exclusively focused on the circulating total 25-hydroxyvitamin D (25(OH)D) as a biomarker of vitamin D status, and have found the causal association between 25(OH)D and the risk of multiple sclerosis (MS). However, it currently remains unclear about the causal association of the 25(OH)D subtypes including 25(OH)D3 and C3-epi-25(OH)D3, as well as calcium with the risk of MS.
Methods
We performed a two-sample MR study to evaluate the causal association of circulating total 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3, and calcium with the risk of MS using large-scale genome-wide association studies (GWAS) datasets from total 25(OH)D (n = 417,580), 25(OH)D3 (n = 40,562), C3-epi-25(OH)D3 (n = 40,562), calcium (n = 305,349), and MS (14,802 MS and 26,703 controls). We selected five MR methods including inverse-variance weighted (IVW), simple median, weighted median, MR-Egger, MR-PRESSO (Mendelian Randomization Pleiotropy Residual Sum and Outlier), and contamination mixture method.
Results
IVW showed that the genetically increased circulating 25(OH)D level (OR = 0.81, 95% CI: 0.70–0.94, P = 4.00E-03), circulating 25(OH)D3 level (OR = 0.85, 95% CI: 0.76–0.95, P = 5.00E-03), and circulating C3-epi-25(OH)D3 level (OR = 0.85, 95% CI: 0.74–0.98, P = 2.30E-02) were causally associated with reduced risk of MS. However, IVW showed no causal association between circulating calcium level and the risk of MS with OR = 2.85, 95% CI: 0.42–19.53, P = 2.85E-01.
Conclusions
Our current findings together with evidence from other MR studies support the use of vitamin D but not calcium supplementation for the prevention of MS.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a neuroinflammatory and neurodegenerative disease in the central nervous system [1]. Vitamin D deficiency is a candidate risk factor for kinds of adverse health outcomes. Observational studies have showed that vitamin D deficiency is associated with increased MS risk [2, 3], and high circulating levels of vitamin D could reduce the risk of MS [4, 5]. Until recently, Mendelian randomization (MR) studies and genetic risk scores (GRS) further supported the causal association between circulating vitamin D levels and the risk of MS [6,7,8,9,10,11,12,13,14]. Mokry and colleagues selected four genetic variants (rs2282679, rs12785878, rs10741657 and rs6013897) as the instrumental variables [7]. Rhead and colleagues selected three genetic variants (rs2282679, rs2060793, and rs3829251) as the instrumental variables [8]. Gianfrancesco and colleagues constructed the GRS using 3 genetic variants including rs2282679, rs2060793, and rs3829251 [9]. Jacobs and colleagues conducted the MR analysis using 6 genetic variants including rs10741657, rs12785878, rs17216707, rs3755967, rs7979805 and rs8018720 [10]. Harroud and colleagues selected 88 independent genetic variants in their MR study [11]. Wang and colleagues selected 20 most effective and independent genetic variants [13]. Vandebergh and colleagues selected 5, 6, 70, and 104 genetic variants from four circulating vitamin D GWAS datasets [14]. Importantly, all these MR studies and GRS show that genetically lowered circulating vitamin D level is causally associated with increased risk of MS [6,7,8,9,10,11,12,13,14].
However, these studies evaluating the causal association between circulating vitamin D levels and the risk of MS exclusively focused on the total 25-hydroxyvitamin D (25(OH)D) as a biomarker of vitamin D status [6,7,8,9,10,11,12,13]. In fact, the total 25(OH)D is the combination of 25(OH)D3, C3-epi-25(OH)D3 (epimeric form of 25(OH)D3), and 25(OH)D2 [15]. 25(OH)D3 is the major component of total 25(OH)D [15]. The European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct case-cohort study identified the mean concentrations (SD) of total 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3, and 25(OH)D2 to be 41.1 (17.2), 40.7 (17.3), 2.13 (1.31), and 8.16 (6.52) nmol/L, respectively [15]. Until now, it remains unclear about the causal association of circulating 25(OH)D3 or C3-epi-25(OH)D3 with the risk of MS.
Meanwhile, vitamin D is an important regulator of serum calcium, and work together with serum calcium in many biological processes [16, 17]. It is recommended that the daily calcium intake is 1000 to 1200 mg [18]. However, diet calcium intake could not achieve this amount. Hence, vitamin and mineral supplements have a large worldwide market, especially in North America and Europe [19]. Evidence shows that calcium supplements are very common in 43% of people (about 70% of older women) in the United States [18, 19]. A population-based cohort study in 475,255 UK Biobank participants (median age 58 years, 55.8% women) indicated that 33,437 (7.04%) participants reported taking calcium supplements; 19,089 (4.02%) vitamin D; and 10,007 (2.11%) both [20]. Importantly, MR study indicated that high genetically determined lifelong circulating 25(OH)D levels were associated with higher calcium levels [21]. However, it currently remains unclear about the causal association between circulating calcium level and the risk of MS.
Until now, large-scale genome-wide association studies (GWAS) have been conducted to identify novel genetic variant associated with circulating total 25(OH)D [22], 25(OH)D3 [23], C3-epi-25(OH)D3 [23], calcium [24], and MS [25]. Therefore, the publicly available GWAS datasets provide strong support to determine the causal association of circulating vitamin D metabolites and calcium with the risk of MS. Here, we performed a MR study to investigate the causal association of circulating total 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3, and calcium with the risk of MS using multiple large-scale GWAS datasets.
Methods
Study design
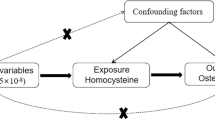
This study is based on large-scale GWAS summary datasets from circulating total 25(OH)D [22], 25(OH)D3 [23], C3-epi-25(OH)D3 [23], calcium [24], and MS [25]. All participants have provided informed consent in the original studies [22,23,24,25]. MR is based on three principal assumptions: (1) genetic variants as the instrumental variables should be significantly associated with the exposure (such as circulating calcium levels) (assumption 1) [26]; (2) genetic variants should not be associated with the confounders of an outcome (MS) (assumption 2) [26]; (3) genetic variants should affect the risk of the outcome (MS) only through the exposure (circulating calcium levels) (assumption 3) [26]. Both the assumption 2 and 3 are defined as the independence from pleiotropy [26]. We provide a flowchart about our MR study design, as described in Fig. 1.
Circulating 25(OH)D genetic variants
Revez and colleagues performed a large-scale GWAS in 417,580 European UK Biobank participants, and identified 143 independent loci including 20 indels and 123 SNPs associated with total 25(OH)D at the genome-wide significance threshold (P < 5.00E-08) [22]. Total 25(OH)D including 25(OH)D3 and 25(OH)D2 was measured using chemiluminescence immunoassay [22]. Revez and colleagues applied a rank-based inverse-normal transformation to the 25(OH)D levels by adjusting for some key covariates including age at time of assessment, sex, assessment month, assessment centre, supplement-intake information, genotyping batch and the first 40 ancestry PCs [22]. The median, mean and interquartile range for 25OHD concentration are 47.9, 49.6, 33.5–63.2 nmol/L [22]. Here, we selected the 123 SNPs as the potential instrumental variables, and provided their detailed information in Supplementary Table 1.
Circulating 25(OH)D3 and C3-epi-25(OH)D3 genetic variants
Zheng and colleagues conducted a meta-analysis of GWAS for 25(OH)D3 and C3-epi-25(OH)D3 in 40,562 participants of European descent including EPIC–InterAct study, EPIC-Norfolk study, EPIC-CVD study, Ely study, and the SUNLIGHT consortium [23]. The 25(OH)D3 and C3-epi-25(OH)D3 metabolites were measured using liquid chromatography–tandem mass spectrometry (LC-MS/MS) [23]. Zheng and colleagues identified 7 genetic variants associated with 25(OH)D3 and 3 genetic variants associated with C3-epi-25(OH)D3 at the genome-wide significance threshold (P < 5.00E-08) [23]. Zheng and colleagues calculated the standardised residuals of natural-log transformed 25(OH)D metabolites by adjusting for age, sex, BMI, season of blood collection, and study centre [23]. Here, we selected these 7 and 3 genetic variants including 8 unique genetic variants as the potential instrumental variables, and provided their detailed information in Supplementary Table 2. Meanwhile, 7 of these 8 unique genetic variants were also significantly associated with total 25(OH)D as provided in Supplementary Table 3, which indicated that these variants were not specific for 25(OH)D metabolites.
Circulating calcium genetic variants
A large-scale GWAS using 305,349 individuals from the UK Biobank had identified 208 independent variants including 32 insertion–deletion mutations (indels), and 176 single nucleotide polymorphisms (SNPs) associated with serum calcium levels at the genome-wide significance threshold (P < 5.00E-08) [24]. These 208 variants could explain 5.8% of the total variance of total serum calcium [24]. Here, we selected these 176 SNPs as the potential instrumental variables, and provided their detailed information in Supplementary Table 4.
MS GWAS dataset
The MS GWAS dataset is from a large-scale meta-analysis of MS GWAS datasets from the International Multiple Sclerosis Genetics Consortium (IMSGC) including 47,429 MS and 68,374 controls [25]. However, only the MS GWAS dataset from the IMSGC discovery stage is publicly available [25]. In brief, IMSGC discovery stage consisted of 14,802 MS cases and 26,703 controls from 15 MS GWAS datasets including GeneMSA DU, GeneMSA SW, GeneMSA US, IMSGC, BWH/MIGEN, ANZ, Berkeley, Rotterdam, UK, CE, Medi, Nordic, US, AUS, and FINLAND [25]. More detailed information about the MS GWAS dataset in IMSGC discovery stage is provided in Table 1.
Establishing the Wald estimator
For each genetic variant Gj (j = 1, …, k), we assume that its beta coefficient \(\hat \beta _{Xj}\) and standard error \(se( {\hat \beta _{Xj}})\) correspond to the associations of each genetic variant with circulating total 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3, and calcium. We then aligned the effect alleles of circulating total 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3, and calcium genetic variants in MS GWAS dataset, and extracted the summary results including the odds ratios (ORs) and the P values. We further translated the ORs and the P values to the beta coefficients and their standard errors corresponding to the associations of each genetic variant Gj (j = 1, …, k) with MS (\(\hat \beta _{Yj}\), and \(se( {\hat \beta _{Yj}})\)). For a given genetic variant, the causal effect of circulating total 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3, and calcium on MS can be estimated by a Wald estimator \(\hat \theta _j\frac{{\hat \beta _{Yj}}}{{\hat \beta _{Xj}}}\) and its approximate variance \(v_j\frac{{se( {\hat \beta _{Yj}})^2}}{{\hat \beta _{Xj}^2}}\) [27].
MR analysis
We selected five methods to conduct the MR analysis including inverse-variance weighted (IVW) [28], weighted median [28], MR-Egger [27], MR-PRESSO (Mendelian Randomization Pleiotropy RESidual Sum and Outlier) [29], and contamination mixture method [30]. IVW is a main and popular MR approach, it assumes that there is no horizontal pleiotropy and all genetic variants are valid instrumental variables, and then combines the single Wald estimates into an overall estimate [28, 30]. MR-Egger could test the directional pleiotropy using MR-Egger intercept test, and correct for the presence of pleiotropy [27]. If the intercept is 0, MR-Egger is the same as IVW [27]. Median-based methods consist of simple median and weighted median [28]. The simple median assumes that at least 50% of genetic variants are valid instrumental variables [28]. The weighted median assumes that at least 50% of the weight is from the valid instrumental variables [28, 31]. Importantly, both simple median and weighted median provide a consistent estimate of causal effect [28]. MR-PRESSO consists of three functions including (1) evaluating the horizontal pleiotropy (MR-PRESSO global test); (2) correcting for the horizontal pleiotropy via outlier removal (MR-PRESSO outlier test); (3) testing the significant distortion in the causal estimates before and after outlier removal (MR-PRESSO distortion test) [29]. Contamination mixture method is newly developed method for MR analysis with hundreds of genetic variants [30]. It performs MR robustly and efficiently even in the presence of invalid instrumental variables [30].
The OR and 95% confidence interval (CI) of MS corresponds to per unit increase in rank-based inverse normal transformed 25OHD levels, per unit increase in natural-log transformed 25(OH)D metabolites (25(OH)D3 and C3-epi-25(OH)D3), and per 1 standard deviation (SD) increase in calcium. All the statistical tests were completed using three R Packages ‘MendelianRandomization’ and ‘MR-PRESSO’, respectively [29, 32]. The threshold of statistically significant association is a Bonferroni corrected significance P < 0.05/4 = 0.0125. The threshold of suggestive association is P < 0.05.
Power analysis
The proportion of exposure variance (R2) explained by the instrumental variables can be estimated as the following formula
Where βi is the effect size (beta coefficient) for SNPi, se (βi) is the standard error for SNPi, K is the number of genetic variants, and N is the sample size [33]. The strength of the instrumental variables was evaluated using the first-stage F-statistic [34, 35].
A common threshold is F > 10 to avoid bias in MR studies [36]. Using the proportion of variance of the R2, the total number of individuals in the analysis, and the proportion of cases in the study, we calculated the statistical power using the web-based tool mRnd, and a two-sided type-I error rate 0.05 [37].
Results
Causal association between circulating 25(OH)D and MS
Using 123 circulating 25(OH)D genetic variants, we successfully extracted their corresponding summary statistics in MS GWAS dataset for 106 genetic variants. 17 genetic variants are not available in MS GWAS dataset, most of which have the minor allele frequency < 5%. Therefore, our MR analysis is based on these 106 genetic variants. For ambiguous palindromic variants (i.e., with alleles either A/T or C/G), we selected their proxy variants using HaploReg v4.1 based on linkage disequilibrium information from 1000 Genomes Project (CEU) with r2 > = 0.8 [38]. IVW showed that the genetically increased circulating 25(OH)D level was causally associated with reduced risk of MS (OR = 0.81, 95% CI: 0.70–0.94, P = 4.00E-03). Interestingly, weighted median, MR-Egger, MR-PRESSO and contamination mixture method further supported the causal association of circulating 25(OH)D level with the risk of MS, and had the consistent estimates with the IVW estimate in terms of direction and magnitude, as provided in Table 2. MR-Egger intercept test did not identify evidence of pleiotropy with intercept = 0, P = 0.91. MR-PRESSO Global Test showed evidence of pleiotropy with P = 1.00E-03, and MR-PRESSO Outlier-corrected test still supported these above findings, as provided in Table 2. The individual causal estimates about the causal association of circulating 25(OH)D levels with the risk of MS using IVW method are provided in Fig. 2.
Causal association between circulating 25(OH)D3 and MS
Of the selected 7 circulating 25(OH)D3 genetic variants, 6 genetic variants excluding rs116970203 are available in MS GWAS dataset. rs116970203 is a rare genetic variant with minor allele frequency 2% [23]. For rs116970203, we identified its proxy variants including rs117621176, rs117361591, rs117913124 using HaploReg v4.1 linkage disequilibrium information from 1000 Genomes Project (CEU) with r2 > = 0.8 [38]. However, these proxies are not available in MS GWAS dataset. Therefore, our MR analysis is based on these 6 genetic variants. IVW showed that the genetically increased circulating 25(OH)D3 level was causally associated with reduced risk of MS (OR = 0.85, 95% CI: 0.76–0.95, P = 5.00E-03). Interestingly, weighted median, MR-PRESSO and contamination mixture method further supported the causal association of circulating 25(OH)D3 level with the risk of MS, and had the consistent estimates with the IVW estimate in terms of direction and magnitude, as provided in Table 2. Meanwhile, we identified no evidence of pleiotropy using MR-Egger intercept test (intercept = −0.016, P = 0.247) and MR-PRESSO Global Test (P = 0.186). The individual causal estimates about the causal association of circulating 25(OH)D3 levels with the risk of MS using IVW method are provided in Fig. 3.
Causal association between circulating C3-epi-25(OH)D3 and MS
Using 3 circulating C3-epi-25(OH)D3 genetic variants, we extracted their corresponding summary statistics in MS GWAS dataset. IVW showed suggestive causal association between genetically increased circulating C3-epi-25(OH)D3 level and reduced risk of MS (OR = 0.85, 95% CI: 0.74–0.98, P = 2.30E-02). Meanwhile, the estimates from weighted median and contamination mixture method were consistent with the IVW estimate in terms of direction and magnitude, although lack of significant association, as provided in Table 2. MR-Egger intercept test indicated no evidence of pleiotropy with intercept = −0.050, P = 0.501. Supplementary Fig. 1 provides the individual estimates about the causal effect of circulating C3-epi-25(OH)D3 on MS using IVW method.
Causal association between circulating calcium and MS
Of the 176 circulating calcium genetic variants, we extracted the summary statistics for 149 common genetic variants in MS GWAS dataset. IVW showed no causal association between circulating calcium level and the risk of MS with OR = 2.85, 95% CI: 0.42–19.53, P = 2.85E-01. Meanwhile, weighted median, MR-Egger, MR-PRESSO, and contamination mixture method suggested no causal association of circulating calcium level with the risk of MS, as provided in Table 2. MR-Egger intercept test indicated no evidence of pleiotropy with intercept = 0.002, P = 0.608. MR-PRESSO Global Test showed evidence of pleiotropy with P < 5E-04. However, MR-PRESSO Outlier-corrected test still reported negative findings, as provided in Table 2. Supplementary Fig. 2 provides the individual estimates about the causal effect of serum calcium on MS using IVW method.
Power analysis
The selected genetic variants explain 4.85%, 3.75%, 0.66%, and 4.42% of the variance in circulating 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3 and calcium, respectively. Meanwhile, these genetic variants have strong strength as the instrumental variables with F > = 89.82. The sample size for the MS GWAS is 41505, and the proportion of MS is 0.36. Using mRnd, our MR study had 80% power to detect the risk of MS with OR < = 0.88, < = 0.86, < = 0.69, > = 1.14 per SD increase in circulating 25(OH)D, 25(OH)D3, C3-epi-25(OH)D3 and calcium, respectively. Table 3 provides the detailed power analysis results.
Discussion
Until now, the causal association between circulating vitamin D level and the risk of MS has been well established [6,7,8,9,10,11,12,13]. However, these studies exclusively selected the circulating total 25(OH)D as a biomarker of vitamin D status [6,7,8,9,10,11,12,13]. It currently remains unclear about the causal association of the 25(OH)D subtypes including 25(OH)D3 and C3-epi-25(OH)D3 with the risk of MS. Here, we investigated the causal association of circulating total 25(OH)D, 25(OH)D3, and C3-epi-25(OH)D3 with the risk of MS using a MR design. We found significant or suggestive causal association of the increased total 25(OH)D, 25(OH)D3, and C3-epi-25(OH)D3 with the reduced risk of MS. Our results are consistent with previous findings that genetically increased circulating total 25(OH)D level was causally associated with increased risk of MS [6,7,8,9,10,11,12,13]. In addition to circulating total 25(OH)D level, we highlighted for the first time that genetically increased circulating 25(OH)D3 and C3-epi-25(OH)D3 were also causally associated with increased risk of MS, as provided in Table 2.
Although these interesting findings, current randomised controlled trials (RCTs) have not provided clear beneficial effects of vitamin D supplementation on MS risk [39,40,41,42]. In 2020, clinical and radiographical findings from EVIDIMS study (Efficacy of Vitamin D Supplementation in Multiple Sclerosis (NCT01440062) did not support nor disprove a therapeutic benefit of high-dose vitamin D supplementation (20,400 IU)/day low-dose (400 IU)/day cholecalciferol supplementation on MS for 18 months [43]. Interestingly, MS patients with higher 25(OH)D levels had lower T2 weighted lesion count and lower Expanded Disability Status Scale (EDSS) score [44]. Meanwhile, there are some promising results young MS patients early after initial diagnosis [40]. A meta-analysis of 12 clinical trials indicated that vitamin D supplementation might be beneficial in preventing MS relapse rates and new radiological signs [42, 45].
Until recently, Galoppin and colleagues have discussed several mechanisms by which 25(OH)D may decrease the risk for MS [46]. First, 25(OH)D has biological effects on immune cells. vitamin D involves immunomodulation by decreasing differentiation of effector T and B cells, and modulates innate immune cells including macrophages, monocytes and dendritic cells [46]. Second, 25(OH)D has biological effects on blood–brain barrier (BBB) function by reducing immune cell trafficking [46]. Third, 25(OH)D reduces microglial and astrocytic activation in the central nervous system [46].
Generally, C3-epi-25(OH)D3 accounts for a significant portion of total 25(OH)D detected in maternal and neonatal umbilical sera [47], and has very low concentration in general population [15]. Interestingly, 25(OH)D3 supplementation increases maternal-fetal C3-epi-25(OH)D3. Importantly, C3-epi-25(OH)D3 increases with increasing 25(OH)D levels and shows a high degree of tracking over time [48]. We consider that the positive association between C3-epi-25(OH)D3 and 25(OH)D may explain why a higher circulating level of C3-epi-25(OH)D3 also reduces the risk of MS in the general population.
It is reported that 25(OH)D regulates the calcium, and work together with calcium in many biological processes [16, 17, 49]. Importantly, MR study supported the causal association between circulating 25(OH)D level and calcium level [21]. Therefore, we further conducted a MR analysis to evaluate the causal association between circulating calcium level and the risk of MS. However we did not find any evidence supporting the causal association. On the one hand, our current findings indicate no beneficial effect of genetically predicted serum calcium on MS risk. On the other hand, our findings show no evidence for harmful effects of genetically predicted serum calcium effects on MS risk. these findings may further suggest that high calcium intake from diet or supplements may not contribute to reduce or increase the risk of MS. It should be pointed out that the circulating calcium level is measured in the general population including 305,349 individuals from the UK Biobank [24]. Therefore, our findings may only reflect the effects of lifelong genetically increased circulating calcium level on MS in the general population, but not in MS persons with osteoporosis.
It has been widely reported that bone mineral density (BMD) is reduced in MS patient, and MS patients have increased osteoporosis risk compared with general population [50,51,52]. Bazelier and colleagues conducted a population-based cohort study in UK General Practice Research Database to estimate the relative and absolute risk of fracture in patients with MS using 5565 MS patients and 33,360 population-based controls [51]. Bazelier and colleagues found 2.79-fold increased risk of hip fracture and 1.35-fold increased risk of osteoporotic fracture in MS compared with controls [51]. Bisson and colleagues analyzed the population-based administrative data from 783 MS cases and 3915 controls to compare measures of BMD in MS and controls [52]. Bisson and colleagues found that the average femoral BMD was lower in MS cases compared with matched controls [52]. The prevalence of osteoporosis across BMD sites was higher in MS (16–26%) compared with matched controls (6–15%) [52]. MS patients had 2.41-fold increased risk of osteoporosis [52].
Until recently, several studies have determined whether increased circulating calcium level could improve BMD and reduce the osteoporotic fractures in the general populations including individuals with normal calcium levels using MR design [53,54,55,56]. Cerani and colleagues performed a MR analysis using large-scale serum calcium GWAS dataset (n = 61,079), heel BMD GWAS dataset (n = 426,824), and fracture GWAS dataset (76,549 cases and 470,164 controls) [53]. They found that genetically increased circulating calcium levels in individuals with normal calcium levels could not increase heel BMD, and could not protect these individuals against fracture [53]. In 2020, Li and colleagues conducted a MR study using large-scale circulating calcium (n = 39,400) and whole-body BMD (n = 66,628) GWAS datasets [54]. They found that genetically increased circulating calcium level was associated with reduced whole-body BMD by adjusting the serum parathyroid hormone, 25(OH)D, and phosphate [54]. Qu and colleagues found that serum calcium levels were inversely associated with lumbar spine BMD (n = 28,498) [55]. Therefore, our finding is comparable to recent MR studies evaluating the effect of circulating calcium level on BMD, which highlighted that genetically increased circulating calcium level was not always better.
Our MR analysis may have several strengths. First, we selected large-scale circulating total 25(OH)D (417,580 individuals) [22], 25(OH)D3 (40,562 participants) [23], C3-epi-25(OH)D3 (40,562 participants) [23], calcium (305,349 individuals) [24], and MS (14,802 MS cases and 26,703 controls) [25] GWAS datasets, which may contribute to provide ample power. Second, all the individuals from these above GWAS datasets are of the European descent, which may contribute to reduce the influence population stratification [57, 58]. Third, more additional genetic variants are selected as the potential instrumental variables compared with previous studies [6,7,8,9,10,11,12,13]. Fourth, we selected two statistical methods including MR-Egger intercept test and MR-PRESSO test to conduct the pleiotropy analysis, which ensure the selected genetic variants to meet the MR assumptions. Fifth, we selected five different MR methods including IVW, weighted median, MR-Egger, MR-PRESSO, and contamination mixture method to ensure the precision of the causal estimate.
Meanwhile, our MR study may have some limitations. First, we only selected one large scale GWAS dataset for MS. Until now, an independent replication dataset with a similar sample size was not publicly available. Second, we could not completely exclude additional confounders. Until now, it is impossible to fully rule out pleiotropy [59, 60]. Some selected genetic variants as the instrumental variables may still have pleiotropic effects, as stated in an editorial regarding vitamin D and CVD risk [61]. Therefore, our current MR study may not provide absolute causality. Third, we limit our MR analysis in individuals of European ancestry. However, the causal association may vary in different ancestries. Fourth, only 3 genetic variants associated with C3-epi-25(OH)D3 at the genome-wide significance threshold (P < 5.00E-08) only explain 0.66% of the variance in circulating C3-epi-25(OH)D3 level. Our MR study only had 80% power to detect the risk of MS with OR < = 0.69 per SD increase in circulating C3-epi-25(OH)D3 level.
Fifth, Yuan and colleagues conducted a MR analysis to evaluate the causal associations of 65 possible risk factors including calcium with the risk of MS [62]. They selected 7 calcium genetic variants as the instrumental variables, and found no significant association between calcium and MS with precise confidence intervals using IVW (OR = 0.78, 95% CI: 0.33–1.83, P = 0.564), weighted median (OR = 0.88, 95% CI: 0.50–1.54, P = 0.644), MR-PRESSO (OR = 0.98, 95% CI: 0.54–1.77, P = 0.940) [62]. Our current findings are consistent with Yuan and colleagues, but with broad confidence intervals, as provided in Table 2. Importantly, Young and colleagues also found broad confidence intervals when they investigated the causal association of serum calcium with ECG markers of ventricular depolarization and repolarization [24]. We consider that these may be caused by the validity of the instrumental variables for calcium from the UK Biobank. Therefore, future studies are required to replicate our findings.
Conclusions
We comprehensively evaluated the causal association of genetically determined circulating vitamin D metabolites and calcium with the risk of MS in individuals of European descent. We demonstrate the causal association between genetically increased circulating vitamin D metabolites and the reduced risk of MS. Our current findings together with evidence from other MR studies support the use of vitamin D supplementation for the prevention of MS. However, we demonstrate no causal association between circulating calcium level and the risk of MS. Therefore, high calcium intake from both diet and supplements may not contribute to reduce the risk of MS.
Data availability
All data generated or analyzed during this study are included in this published article and its Additional files. The authors confirm that all data underlying the findings are either fully available without restriction through consortia websites, or may be made available from consortia upon request. International Multiple Sclerosis Genetics Consortium (IMSGC): https://imsgc.net/.
References
Liu G, Zhang F, Jiang Y, Hu Y, Gong Z, Liu S, et al. Integrating genome-wide association studies and gene expression data highlights dysregulated multiple sclerosis risk pathways. Mult Scler. 2017;23:205–12.
Munger KL, Zhang SM, O’Reilly E, Hernan MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62:60–65.
Munger KL, Hongell K, Aivo J, Soilu-Hanninen M, Surcel HM, Ascherio A. 25-Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology 2017;89:1578–83.
Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012;79:2140–5.
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–8.
Harroud A, Richards JB. Mendelian randomization in multiple sclerosis: A causal role for vitamin D and obesity? Mult Scler. 2018;24:80–85.
Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Goltzman D, et al. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2015;12:e1001866.
Rhead B, Baarnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2:e97.
Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology 2017;88:1623–9.
Jacobs BM, Noyce AJ, Giovannoni G, Dobson R. BMI and low vitamin D are causal factors for multiple sclerosis: A Mendelian Randomization study. Neurol Neuroimmunol Neuroinflamm. 2020;7:e662.
Harroud A, Manousaki D, Butler-Laporte G, Mitchell RE, Davey Smith G, Richards JB, et al. The relative contributions of obesity, vitamin D, leptin, and adiponectin to multiple sclerosis risk: A Mendelian randomization mediation analysis. Mult Scler. 2021;27:1994–2000.
Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: Evidence from human studies. Nat Rev Endocrinol. 2022;18:96–110.
Wang R. Mendelian randomization study updates the effect of 25-hydroxyvitamin D levels on the risk of multiple sclerosis. J Transl Med. 2022;20:3.
Vandebergh M, Dubois B, Goris A. Effects of Vitamin D and body mass index on disease risk and relapse hazard in multiple sclerosis: A mendelian randomization study. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1165.
Zheng JS, Imamura F, Sharp SJ, van der Schouw YT, Sluijs I, Gundersen TE, et al. Association of plasma vitamin D metabolites with incident type 2 diabetes: EPIC-InterAct case-cohort study. J Clin Endocrinol Metab. 2019;104:1293–303.
Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–41.
Arnold A, Dennison E, Kovacs CS, Mannstadt M, Rizzoli R, Brandi ML, et al. Hormonal regulation of biomineralization. Nat Rev Endocrinol. 2021;17:261–75.
Kern J, Kern S, Blennow K, Zetterberg H, Waern M, Guo X, et al. Calcium supplementation and risk of dementia in women with cerebrovascular disease. Neurology 2016;87:1674–80.
Zhang FF, Barr SI, McNulty H, Li D, Blumberg JB. Health effects of vitamin and mineral supplements. BMJ. 2020;369:m2511.
Harvey NC, D’Angelo S, Paccou J, Curtis EM, Edwards M, Raisi-Estabragh Z, et al. Calcium and Vitamin D supplementation are not associated with risk of incident ischemic cardiac events or death: Findings from the UK biobank cohort. J Bone Min Res. 2018;33:803–11.
Jian Z, Huang Y, He Y, Jin X, Li H, Li S, et al. Genetically predicted lifelong circulating 25(OH)D levels are associated with serum calcium levels and kidney stone risk. J Clin Endocrinol Metab. 2022;107:e1159–e1166.
Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. 2020;11:1647.
Zheng JS, Luan J, Sofianopoulou E, Sharp SJ, Day FR, Imamura F, et al. The association between circulating 25-hydroxyvitamin D metabolites and type 2 diabetes in European populations: A meta-analysis and Mendelian randomisation analysis. PLoS Med. 2020;17:e1003394.
Young WJ, Warren HR, Mook-Kanamori DO, Ramirez J, van Duijvenboden S, Orini M, et al. Genetically determined serum calcium levels and markers of ventricular repolarization: a mendelian randomization study in the UK Biobank. Circ Genom Precis Med. 2021;14:e003231.
Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019; 365(6460):eaav7188.
Liu G, Zhao Y, Jin S, Hu Y, Wang T, Tian R, et al. Circulating vitamin E levels and Alzheimer’s disease: A Mendelian randomization study. Neurobiol Aging. 2018;72:189 e181–189 e189.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89.
Bowden J, Davey, Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11:376.
Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. 2019;10:486–96.
Yavorska OO, Burgess S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–9.
Shim H, Chasman DI, Smith JD, Mora S, Ridker PM, Nickerson DA, et al. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS One. 2015;10:e0120758.
Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40:740–52.
Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–42.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64.
Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–501.
Ward LD, Kellis M. HaploReg: A resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–934.
Maretzke F, Bechthold A, Egert S, Ernst JB, Melo van Lent D, Pilz S, et al. Role of Vitamin D in preventing and treating selected extraskeletal diseases-an umbrella review. Nutrients. 2020;12:969.
Boltjes R, Knippenberg S, Gerlach O, Hupperts R, Damoiseaux J. Vitamin D supplementation in multiple sclerosis: An expert opinion based on the review of current evidence. Expert Rev Neurother. 2021;21:715–25.
Feige J, Moser T, Bieler L, Schwenker K, Hauer L, Sellner J. Vitamin D supplementation in multiple sclerosis: A critical analysis of potentials and threats. Nutrients. 2020;12:783.
Fatima M, Lamis A, Siddiqui SW, Ashok T, Patni N, Fadiora OE. Therapeutic role of Vitamin D in multiple sclerosis: An essentially contested concept. Cureus 2022;14:e26186.
Dorr J, Backer-Koduah P, Wernecke KD, Becker E, Hoffmann F, Faiss J, et al. High-dose vitamin D supplementation in multiple sclerosis - results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial. Mult Scler J Exp Transl Clin. 2020;6:2055217320903474.
Backer-Koduah P, Bellmann-Strobl J, Scheel M, Wuerfel J, Wernecke KD, Dorr J, et al. Vitamin D and disease severity in multiple sclerosis-baseline data from the randomized controlled trial (EVIDIMS). Front Neurol. 2020;11:129.
McLaughlin L, Clarke L, Khalilidehkordi E, Butzkueven H, Taylor B, Broadley SA. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J Neurol. 2018;265:2893–905.
Galoppin M, Kari S, Soldati S, Pal A, Rival M, Engelhardt B, et al. Full spectrum of vitamin D immunomodulation in multiple sclerosis: Mechanisms and therapeutic implications. Brain Commun. 2022;4:fcac171.
Mao D, Yuen LY, Ho CS, Wang CC, Tam CH, Chan MH, et al. Maternal and Neonatal 3-epi-25-hydroxyvitamin D concentration and factors influencing their concentrations. J Endocr Soc. 2022;6:bvab170.
Kubiak JM, Grimnes G, Cashman KD, Kamycheva E, Dowling K, Skrabakova Z, et al. C3-epimerization of 25-hydroxyvitamin D increases with increasing serum 25-hydroxyvitamin D levels and shows a high degree of tracking over time. Clin Biochem. 2018;54:61–7.
Yuan S, Jiang X, Michaelsson K, Larsson SC. Genetic Prediction of Serum 25-Hydroxyvitamin D, Calcium, and Parathyroid hormone levels in relation to development of type 2 diabetes: A mendelian randomization study. Diabetes Care. 2019;42:2197–203.
Schwid SR, Goodman AD, Puzas JE, McDermott MP, Mattson DH. Sporadic corticosteroid pulses and osteoporosis in multiple sclerosis. Arch Neurol. 1996;53:753–7.
Bazelier MT, van Staa T, Uitdehaag BM, Cooper C, Leufkens HG, Vestergaard P, et al. The risk of fracture in patients with multiple sclerosis: the UK general practice research database. J Bone Min Res. 2011;26:2271–9.
Bisson EJ, Finlayson ML, Ekuma O, Leslie WD, Marrie RA. Multiple sclerosis is associated with low bone mineral density and osteoporosis. Neurol Clin Pr. 2019;9:391–9.
Cerani A, Zhou S, Forgetta V, Morris JA, Trajanoska K, Rivadeneira F, et al. Genetic predisposition to increased serum calcium, bone mineral density, and fracture risk in individuals with normal calcium levels: mendelian randomisation study. BMJ. 2019;366:l4410.
Li GH, Robinson-Cohen C, Sahni S, Au PC, Tan KC, Kung AW, et al. Association of genetic variants related to serum calcium levels with reduced bone mineral density. J Clin Endocrinol Metab. 2020;105:e328–e36.
Qu Z, Yang F, Yan Y, Hong J, Wang W, Li S, et al. Relationship between serum nutritional factors and bone mineral density: A Mendelian randomization study. J Clin Endocrinol Metab. 2021;106:e2434–e43.
Sun JY, Zhang H, Zhang Y, Wang L, Sun BL, Gao F, et al. Impact of serum calcium levels on total body bone mineral density: A mendelian randomization study in five age strata. Clin Nutr. 2021;40:2726–33.
O’Seaghdha CM, Wu H, Yang Q, Kapur K, Guessous I, Zuber AM, et al. Meta-analysis of genome-wide association studies identifies six new Loci for serum calcium concentrations. PLoS Genet. 2013;9:e1003796.
de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49:256–61.
Emdin CA, Khera AV, Natarajan P, Klarin D, Zekavat SM, Hsiao AJ, et al. Genetic association of Waist-to-Hip Ratio With Cardiometabolic Traits, Type 2 Diabetes, and coronary heart disease. JAMA. 2017;317:626–34.
Larsson SC, Burgess S, Michaelsson K. Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA. 2017;318:371–80.
Burgess S, Gill D. Genetic evidence for vitamin D and cardiovascular disease: Choice of variants is critical. Eur Heart J. 2022;43:1740–2.
Yuan S, Xiong Y, Larsson SC. An atlas on risk factors for multiple sclerosis: a Mendelian randomization study. J Neurol. 2021;268:114–24.
Acknowledgements
We thank International Multiple Sclerosis Genetics Consortium (IMSGC) for the GWAS summary statistics.
Funding
This work was supported by funding from the National Natural Science Foundation of China (Grant No. 82071212, and 81901181), Beijing Natural Science Foundation (Grant No. JQ21022), the Mathematical Tianyuan Fund of the National Natural Science Foundation of China (Grant No. 12026414), and Beijing Ten Thousand Talents Project (Grant No. 2020A15). This work was also partially supported by funding from the Science and Technology Beijing One Hundred Leading Talent Training Project (Z141107001514006), the Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150802), the Funds of Academic Promotion Programme of Shandong First Medical University & Shandong Academy of Medical Sciences (No. 2019QL016, No. 2019PT007).
Author information
Authors and Affiliations
Contributions
GYL and YZ conceived and initiated the project. GYL and YZ analyzed the data, and wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This article contains human participants collected by several GWAS. All participants gave informed consent in all the corresponding original studies. Here, we only used the large-scale GWAS summary datasets, and not the individual-level data. Hence, ethical approval was not sought.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Liu, H., Zhang, H. et al. Causal association of genetically determined circulating vitamin D metabolites and calcium with multiple sclerosis in participants of European descent. Eur J Clin Nutr 77, 481–489 (2023). https://doi.org/10.1038/s41430-023-01260-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01260-4