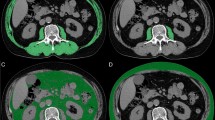
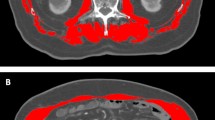
Abstract
Introduction
Gynaecology cancers, including ovarian (OC), endometrial (EC), and cervical (CC), are prevalent with high mortality. Sarcopenia is found in 38.7% of cancer patients, adversely affecting prognosis. Computed tomography (CT) is performed routinely in oncology, yet CT assessments of sarcopenia are not commonly used to measure prognosis. This systematic review and meta-analysis aimed to evaluate the prognostic potential of pre-treatment sarcopenia assessments on overall survival (OS) and progression free survival (PFS) in gynaecology cancer.
Methodology
Four electronic databases were systematically searched from 2000 to May 2020 in English: Ovid Medline, EMBASE, Web of Science, and CINAHL plus. Titles and abstracts were screened, eligible full-texts were reviewed, and data from included studies was extracted. Meta-analyses were conducted on homogenous survival data, heterogenous data were narratively reported.
Results
The initial search yielded 767 results; 27 studies were included in the systematic review (n = 4286), all published between 2015 and 2020. Meta-analysis of unadjusted results revealed a negative effect of pre-treatment sarcopenia on OS in OC (HR: 1.40, 1.20–1.64, p < 0.0001) (n = 10), EC (HR: 1.42, 0.97–2.10, p = 0.07) (n = 4) and CC (HR: 1.10, 0.93–1.31, p = 0.28) (n = 5), and a negative effect on PFS in OC (HR: 1.28, 1.11–1.46, p = 0.0005) (n = 8), EC (HR: 1.51, 1.03–2.20, p = 0.03) (n = 2) and CC (HR: 1.14, 0.85–1.53, p = 0.37) (n = 2). Longitudinal analysis indicated negative effects of muscle loss on survival. Overall, there was a high risk of bias.
Conclusion
Pre-treatment sarcopenia negatively affected survival in gynaecology cancers. Incorporating such assessments into cancer management may be beneficial. Heterogeneity in sarcopenia assessments makes data interpretation challenging. Further research in prospective studies is required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
WCRF (World Cancer Research Fund). Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report; 2018.
Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909–27. https://doi.org/10.1097/AOG.0000000000002580.
Ledford LRC, Lockwood S. Scope and epidemiology of gynecologic cancers: an overview. Semin Oncol Nurs. 2019;35:147–50. https://doi.org/10.1016/j.soncn.2019.03.002.
Billson HA, Holland C, Curwell J, Davey VL, Kinsey L, Lawton LJ, et al. Perioperative nutrition interventions for women with ovarian cancer. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD009884.pub2.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27:e43. https://doi.org/10.3802/jgo.2016.27.e43.
Davidson B. Endometrial cancer: pathology and genetics. Encyclopedia of Cancer. 3rd ed. Editor(s): Paolo Boffetta, Pierre Hainaut. Academic Press; 2018. p. 549–58. https://doi.org/10.1016/B978-0-12-801238-3.65262-5.
Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719–30. https://doi.org/10.1002/ijc.31961.
Bagaria M, Shields E, Bakkum-Gamez JN. Novel approaches to early detection of endometrial cancer. Curr Opin Obstet Gynecol. 2017;29:40–6. https://doi.org/10.1097/GCO.0000000000000332.
Passarello K, Kurian S, Villanueva V. Endometrial cancer: an overview of pathophysiology, management, and care. Semin Oncol Nurs. 2019;35:157–65. https://doi.org/10.1016/j.soncn.2019.02.002.
Elia M. The ‘MUST’ report: nutritional screening of adults: a multidisciplinary responsibility. Reddich: BAPEN; 2003.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon k, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. https://doi.org/10.1016/j.clnu.2016.07.015.
Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91–104. https://doi.org/10.1016/j.critrevonc.2018.05.006.
Sandri M. Protein breakdown in cancer cachexia. Semin Cell Dev Biol. 2016;54:11–19. https://doi.org/10.1016/j.semcdb.2015.11.002.
Rodrigues CS, Lacerda MS, Chaves GV. Patient generated subjective global assessment as a prognosis tool in women with gynecologic cancer. Nutrition. 2015;31:1372–8. https://doi.org/10.1016/j.nut.2015.06.001.
Hertlein L, Kirschenhofer A, Fürst S, Beer D, Göß C, Lenhard M, et al. Malnutrition and clinical outcome in gynecologic patients. Eur J Obstet Gynecol Reprod Biol. 2014;174:137–40. https://doi.org/10.1016/j.ejogrb.2013.12.028.
Cantrell LA, Saks E, Grajales V, Duska L. Nutrition in gynecologic cancer. Curr Obstet Gynecol Rep. 2015;4:265–71. https://doi.org/10.1007/s13669-015-0130-2.
Laky B, Janda M, Bauer J, Vavra C, Cleghorn G, Obermair A. Malnutrition among gynaecological cancer patients. Eur J Clin Nutr. 2007;61:642–6. https://doi.org/10.1038/sj.ejcn.1602540.
Obermair A, Simunovic M, Isenring L, Janda M. Nutrition interventions in patients with gynecological cancers requiring surgery. Gynecol Oncol. 2017;145:192–9. https://doi.org/10.1016/j.ygyno.2017.01.028.
Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla E, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75:199–211. https://doi.org/10.1017/S002966511500419X.
Muscaritoli M, Lucia S, Farcomeni A, Lorusso V, Saracino V, Barone C, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8:79884–96. https://doi.org/10.18632/oncotarget.20168.
Rauh S, Antonuzzo A, Bossi P, Eckert R, Fallon M, Fröbe A, et al. Nutrition in patients with cancer: a new area for medical oncologists? A practising oncologist’s interdisciplinary position paper. ESMO Open. 2018;3:1–4. https://doi.org/10.1136/esmoopen-2018-000345.
Szewczuk M, Gasiorowska E, Matysiak K, Nowak-Markwitz E. The role of artificial nutrition in gynecological cancer therapy. Ginekol Pol. 2019;90:167–72. https://doi.org/10.5603/GP.2019.0027.
Baracos VE. Cancer-associated malnutrition. Eur J Clin Nutr. 2018;72:1255–9. https://doi.org/10.1038/s41430-018-0245-4.
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, et al. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1419–27. https://doi.org/10.1007/s00384-018-3128-1.
Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N. Sarcopenia in patients with colorectal cancer: a comprehensive review. World J Clin Cases. 2020;8:1188–202. https://doi.org/10.12998/wjcc.v8.i7.1188.
Cianci S, Rumolo V, Rosati A, Scaletta G, Alletti SG, Cerentini TM, et al. Sarcopenia in ovarian cancer patients, oncologic outcomes revealing the importance of clinical nutrition: review of literature. Curr Pharm Des. 2019;25:2480–90. https://doi.org/10.2174/1381612825666190722112808.
Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018;37:1101–13. https://doi.org/10.1016/j.clnu.2017.07.010.
Aust S, Knogler T, Pils D, Obermayr E, Reinthaller A, Zahn L, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS ONE. 2015;10:1–14. https://doi.org/10.1371/journal.pone.0140403.
Huang CY, Sun FJ, Lee J. Prognostic value of muscle measurement using the standardized phase of computed tomography in patients with advanced ovarian cancer. Nutrition. 2020a;72:e110642. https://doi.org/10.1016/j.nut.2019.110642.
Ganju RG, TenNapel M, Spoozak L, Chen AM, Hoover A. The impact of skeletal muscle abnormalities on tolerance to adjuvant chemotherapy and radiation and outcome in patients with endometrial cancer. J Med Imaging Radiat Oncol. 2020;64:104–12. https://doi.org/10.1111/1754-9485.12935.
Lee J, Chang CL, Lin J, Bin WMH, Sun FJ, Jan YT, et al. Skeletal muscle loss is an imaging biomarker of outcome after definitive chemoradiotherapy for locally advanced cervical cancer. Clin Cancer Res. 2018;24:5028–36. https://doi.org/10.1158/1078-0432.CCR-18-0788.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. https://doi.org/10.1093/ageing/afy169.
Ubachs J, Ziemons J, Minis-Rutten IJG, Kruitwagen RFPM, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:1165–74. https://doi.org/10.1002/jcsm.12468.
Nakayama N, Nakayama K, Nakamura K, Razia S, Kyo S. Sarcopenic factors may have no impact on outcomes in ovarian cancer patients. Diagnostics. 2019;9:1–9. https://doi.org/10.3390/diagnostics9040206.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. https://doi.org/10.1016/S1470-2045(08)70153-0.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. https://doi.org/10.1016/j.ejca.2015.12.030.
Riley RD, Moons KG, Snell KE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. https://doi.org/10.1136/bmj.k4597.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hayden JA, van der Windt DA, Cartwright JL, Co P. Research and reporting methods annals of internal medicine assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009.
Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Fragkos K, Tsagris M, Frangos C. Exploring the distribution for the estimator of Rosenthal’s ‘fail-safe’ number of unpublished studies in meta-analysis. Commun Stat Theory Methods. 2016 (in press).
Sterne J, Sutton A, Ioannidis J, Terrin N, Jones D, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Ataseven B, Luengo TG, du Bois A, Waltering KU, Traut A, Heitz F, et al. Skeletal muscle attenuation (sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol. 2018;25:3372–9. https://doi.org/10.1245/s10434-018-6683-3.
Bronger H, Hederich P, Hapfelmeier A, Metz S, Noël PB, Kiechle M, et al. Sarcopenia in advanced serous ovarian cancer. Int J Gynecol Cancer. 2017;27:223–32. https://doi.org/10.1097/IGC.0000000000000867.
Conrad LB, Awdeh H, Acosta-Torres S, Conrad SA, Bailey AA, Miller DS, et al. Pre-operative core muscle index in combination with hypoalbuminemia is associated with poor prognosis in advanced ovarian cancer. J Surg Oncol. 2018;117:1020–8. https://doi.org/10.1002/jso.24990.
De Paula NS, Rodrigues CS, Chaves GV. Comparison of the prognostic value of different skeletal muscle radiodensity parameters in endometrial cancer. Eur J Clin Nutr. 2019;73:524–30. https://doi.org/10.1038/s41430-018-0163-5.
Gillen J, Mills KA, Dvorak J, Xheng B, Thai T, Salani R, et al. Imaging biomarkers of adiposity and sarcopenia as potential predictors for overall survival among patients with endometrial cancer treated with bevacizumab. Gynecologic Oncol Rep. 2019;30:100502. https://doi.org/10.1016/j.gore.2019.100502.
Huang CY, Yang YC, Chen TC, Chen JR, Chen YJ, Wu MH, et al. Muscle loss during primary debulking surgery and chemotherapy predicts poor survival in advanced-stage ovarian cancer. J Cachexia Sarcopenia Muscle. 2020b;11:534–46. https://doi.org/10.1002/jcsm.12524.
Kim SI, Kim TM, Lee M, Kim HS, Chung HH, Cho JY, et al. Impact of ct-determined sarcopenia and body composition on survival outcome in patients with advanced-stage high-grade serous ovarian carcinoma. Cancers. 2020;12:1–17. https://doi.org/10.3390/cancers12030559.
Kiyotoki T, Nakamura K, Haraga J, Omichi C, Ida N, Saijo M, et al. Sarcopenia is an important prognostic factor in patients with cervical cancer undergoing concurrent chemoradiotherapy. Int J Gynecol Cancer. 2018;28:168–75. https://doi.org/10.1097/IGC.0000000000001127.
Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142:311–6. https://doi.org/10.1016/j.ygyno.2016.05.027.
Kuroki L, Mangano M, Allsworth J, Menias C, Massad L, Powell M, et al. Sarcopenia: pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol. 2015;22:972–9. https://doi.org/10.1245/s10434-014-4040-8.
Lee J, Lin J, Bin WMH, Chang CL, Jan YT, Chen YJ. Muscle loss after chemoradiotherapy as a biomarker of distant failures in locally advanced cervical cancer. Cancers. 2020;12:1–14. https://doi.org/10.3390/cancers12030595.
Lee J, Lin J, Bin WMH, Jan YT, Chang CL, Huang CY, et al. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J Cachexia Sarcopenia Muscle. 2019;10:814–26. https://doi.org/10.1002/jcsm.12440.
Matsubara Y, Nakamura K, Matsuoka H, Ogawa C, Masuyama H. Pre-treatment psoas major volume is a predictor of poor prognosis for patients with epithelial ovarian cancer. Mol Clin Oncol. 2019;11:376–82. https://doi.org/10.3892/mco.2019.1912.
Matsuoka H, Nakamura K, Matsubara Y, Ida N, Nishida T, Ogawa C, et al. Sarcopenia is not a prognostic factor of outcome in patients with cervical cancer undergoing concurrent chemoradiotherapy or radiotherapy. Anticancer Res. 2019;39:933–9. https://doi.org/10.21873/anticanres.13196.
Nattenmüller J, Rom J, Buckner T, Arvin J, Bau B, Sohn C, et al. Visceral abdominal fat measured by computer tomography as a prognostic factor for gynecological malignancies? Oncotarget. 2018;9:16330–42. https://doi.org/10.18632/oncotarget.24667.
Rodrigues CS, Chaves GV. Skeletal muscle quality beyond average muscle attenuation: a proposal of skeletal muscle phenotypes to predict short-term survival in patients with endometrial cancer. JNCCN. 2018;16:153–60. https://doi.org/10.6004/jnccn.2017.7028.
Rutten IJG, Ubachs J, Kruitwagen RFPM, van Dijk DPJ, Beets-Tan RGH, Massuger LFAG, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017a;43:717–24. https://doi.org/10.1016/j.ejso.2016.12.016.
Rutten IJG, Ubachs J, Kruitwagen RFPM, Beets-Tan RGH, Olde Damink SWM, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle. 2017b;8:630–8. https://doi.org/10.1002/jcsm.12180.
Rutten IJG, van Dijk DPJ, Kruitwagen RFPM, Beets-Tan RGH, Olde Damink SWM, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:458–66. https://doi.org/10.1002/jcsm.12107.
Sanchez M, Castro-Eguiluz D, Luvián-Morales J, Jiménez-Lima R, Aguilar-Ponce JL, Isla-Ortiz D, et al. Deterioration of nutritional status of patients with locally advanced cervical cancer during treatment with concomitant chemoradiotherapy. J Hum Nutr Diet. 2019;32:480–91. https://doi.org/10.1111/jhn.12649.
Staley SA, Tucker K, Newton M, Ertel M, Oldan J, Doherty I, et al. Sarcopenia as a predictor of survival and chemotoxicity in patients with epithelial ovarian cancer receiving platinum and taxane-based chemotherapy. Gynecol Oncol. 2020;156:695–700. https://doi.org/10.1016/j.ygyno.2020.01.003.
Yoshikawa N, Shirakawa A, Yoshida K, Tamauchi S, Suzuki S, Kikkawa F, et al. Sarcopenia as a predictor of survival among patients with organ metastatic cervical cancer. Nutr Clin Pr. 2020;00:1–6. https://doi.org/10.1002/ncp.10482.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin M, McCargar L, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47.
Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. https://doi.org/10.1139/H08-075.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. https://doi.org/10.1016/S1470-2045(10)70218-7.
Rinninella E, Fagotti A, Cintoni M, Raoul P, Scaletta G, Scambia G, et al. Skeletal muscle mass as a prognostic indicator of outcomes in ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2020;30:654–63. https://doi.org/10.1136/ijgc-2020-001215.
McSharry V, Mullee A, McCann L, Rogers AC, McKiernan M, Brennan DJ. The impact of sarcopenia and low muscle attenuation on overall survival in epithelial ovarian cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2020;10:1165–74. https://doi.org/10.1245/s10434-020-08382-0.
Allanson ER, Peng Y, Choi A, Hayes S, Janda M, Obermair A. A systematic review and meta-analysis of sarcopenia as a prognostic factor in gynecological malignancy. Int J Gynecol Cancer. 2020. https://doi.org/10.1136/ijgc-2020-001678.
Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145:102839. https://doi.org/10.1016/j.critrevonc.2019.102839.
Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, et al. Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018;24:278–84. https://doi.org/10.1111/tbj.12952.
Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Color Dis. 2015;17:20–26. https://doi.org/10.1111/codi.12805.
Sebro R, O’Brien L, Torriani M, Bredella MA. Assessment of trunk muscle density using CT and its association with degenerative disc and facet joint disease of the lumbar spine. Skelet Radio. 2016;45:1221–6. https://doi.org/10.1007/s00256-016-2405-8.
Daly LE, Ní Bhuachalla ÉB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. 2018;9:315–25. https://doi.org/10.1002/jcsm.12267.
Bozzetti F. Chemotherapy-induced sarcopenia. Curr Treat Options Oncol. 2020;21. https://doi.org/10.1007/s11864-019-0691-9.
Hojan K, Milecki P, Molinska-Glura M, Roszak A, Leszczynski P. Effect of physical activtiy on bone strength and body composition in breast cancer premenopausal women during endocrine therapy. Eur J Phys Rehab Med. 2013;49:331–9.
Tan BHL, Birdsell LA, Martin L, Baracos VE, Fearon KCH. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–9. https://doi.org/10.1158/1078-0432.CCR-09-1525.
Trivella M. Systematic reviews of prognostic factor studies (section: estimating the hazard ratio) [DPhil]. University of Oxford; 2006.
Acknowledgements
ES would like to acknowledge her affiliation with the Division of Medicine, University College London, and Dr Marialena Trivella who provided invaluable support with the statistical analysis. No financial assistance was received.
Author information
Authors and Affiliations
Contributions
ES led the review, was responsible for designing the review protocol, writing the protocol and report, conducting the search, screening eligible studies, extracting and analysing data, conducting meta-analysis, deriving all tables and figures. MP supported the research process, made critical comments that helped in the interpretation of results, supported in writing sections of the report, and reviewed the final report. SDC provided expert clinical advice and reviewed the final report. KF reviewed the final report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sutton, E.H., Plyta, M., Fragkos, K. et al. Pre-treatment sarcopenic assessments as a prognostic factor for gynaecology cancer outcomes: systematic review and meta-analysis. Eur J Clin Nutr 76, 1513–1527 (2022). https://doi.org/10.1038/s41430-022-01085-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01085-7