Abstract
Background/objectives
Nighttime eating (NE) behavior has a genetic component and predicts weight gain. We hypothesized that some genetic variants, which affect NE would also show an effect on body mass index (BMI). We aimed to determine which known BMI variants associate with NE in Southwestern American Indians (SWAIs), who are at elevated risk for obesity.
Methods
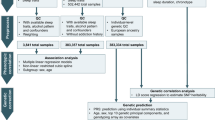
Known BMI variants from the GIANT-UK Biobank meta-analysis (N = 700,000) were analysed in SWAIs characterized for NE during an inpatient 3-day protocol. Variants were analysed for association with NE using whole-genome sequence data from 50 SWAIs (23 cases and 27 controls) and selected variants were genotyped in an additional 32 SWAIs (13 NE cases and 19 controls). Variants associated with NE in a meta-analysis of the two SWAI samples were further analysed for association with nightly caloric intake and functionality in hypothalamus, pituitary, and adrenal tissues.
Results
Variants were identified where the allele that associated with increased BMI in the GIANT-UK Biobank meta-analysis (P ≤ 1 × 10−8) also had a P < 0.01 for increased NE in the SWAI meta-analysis. These variants were captured by six tagSNPs. Comparison of the nightly calorie intake by genotype and eQTL data from relevant tissues highlighted rs3753612 upstream of HCRTR1.
Conclusions
Our strategy led to the HCRTR1 locus, which has previously been linked to sleep regulation and feeding. Although this is an intriguing candidate gene for NE, further studies in larger samples and different populations are required to validate the role of HCRTR1 in NE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206.
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ~700,000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–9.
Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome; a pattern of food intake among certain obese patients. Am J Med. 1955;19:78–86.
Morse SA, Ciechanowski PS, Katon WJ, Hirsch IB. Isn’t this just bedtime snacking? The potential adverse effects of night-eating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care. 2006;29:1800–4.
O’Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS, et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res. 2004;12:1789–96.
Birketvedt GS, Florholmen J, Sundsfjord J, Osterud B, Dinges D, Bilker W, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA. 1999;282:657–63.
Stunkard AJ, Allison KC, O’Reardon JP. The night eating syndrome: a progress report. Appetite. 2005;45:182–6.
Allison KC, Lundgren JD, O’Reardon JP, Geliebter A, Gluck ME, Vinai P, et al. Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord. 2010;43:241–7.
Stunkard AJ, Allison KC, Geliebter A, Lundgren JD, Gluck ME, O’Reardon JP. Development of criteria for a diagnosis: lessons from the night eating syndrome. Compr Psychiatry. 2009;50:391–9.
Adami GF, Campostano A, Marinari GM, Ravera G, Scopinaro N. Night eating in obesity: a descriptive study. Nutrition. 2002;18:587–9.
Gluck ME, Geliebter A, Satov T. Night eating syndrome is associated with depression, low self-esteem, reduced daytime hunger, and less weight loss in obese outpatients. Obes Res. 2001;9:264–7.
Cerú-Björk C, Andersson I, Rössner S. Night eating and nocturnal eating-two different or similar syndromes among obese patients? Int J Obes Relat Metab Disord. 2001;25:365–72.
Allison KC, Wadden TA, Sarwer DB, Fabricatore AN, Crerand CE, Gibbons LM, et al. Night eating syndrome and binge eating disorder among persons seeking bariatric surgery: prevalence and related features. Surg Obes Relat Dis. 2006;2:153–8.
Vander Wal JS. Night eating syndrome: a critical review of the literature. Clin Psychol Rev. 2012;32:49–59.
Gluck ME, Venti CA, Salbe AD, Krakoff J. Nighttime eating: commonly observed and related to weight gain in an inpatient food intake study. Am J Clin Nutr. 2008;88:900–5.
Gluck ME, Venti CA, Salbe AD, Votruba SB, Krakoff J. Higher 24-h respiratory quotient and higher spontaneous physical activity in nighttime eaters. Obesity. 2011;19:319–23.
Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–7.
Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86:625–32.
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2.
Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36:981–90.
Allison KC, Goel N, Ahima RS. Delayed timing of eating: Impact on weight and metabolism. Curr Obes Rep. 2014;3:91–100.
Lundgren JD, Allison KC, Stunkard AJ. Familial aggregation in the night eating syndrome. Int J Eat Disord. 2006;39:516–8.
Ferraro R, Boyce VL, Swinburn B, De GM, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53:1368–71.
Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr. 2009;91:343–8.
Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav. 1998;63:919–28.
GTEx Consortium. Genetic effects on gene expression across human tissues. Nature. 2017;550:204–13.
Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81.
Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–14.
Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008;94:169–77.
Gluck M, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66:876–81.
Birketvedt GS, Sundsfjord J, Florholmen JR. Hypothalamic-pituitary-adrenal axis in the night eating syndrome. Am J Physiol Endocrinol Metab. 2002;282:E366–9.
Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol Rev. 2006;58:46–57.
Acknowledgements
We thank the volunteers for their participation in the study. This work was supported by the Intramural Research Program of the NIDDK, NIH. We utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Funding
This work was supported by the Intramural Research Program of the National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Author information
Authors and Affiliations
Contributions
CK and LJB designed the study. CK, MEG, and SBV generated and analysed the data. EJS contributed in data collection and entry. JK, PC, CB, PP, and LJB contributed in consultation regarding data analysis and interpretation of the results. MT contributed in the interpretation of the results and the preparation of figures. CK, MT, and LJB wrote the paper. All authors have revised the paper and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Köroğlu, Ç., Gluck, M.E., Traurig, M. et al. Assessing established BMI variants for a role in nighttime eating behavior in robustly phenotyped Southwestern American Indians. Eur J Clin Nutr 74, 1718–1724 (2020). https://doi.org/10.1038/s41430-020-0654-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-0654-z