Abstract
Objectives
Coronary artery calcification (CAC) can reliably predict cardiovascular events. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are thought to inhibit vascular calcification on a cellular level and in animal models, however, the correlation in humans is controversial.
Methods
In symptomatic patients, CAC was quantified according to Agatstons’ method using non-contrast coronary CT. We assessed the association of EPA and DHA with early-onset coronary atherosclerosis, defined as presence of CAC above the 75th Agatston-Score (AS) percentile in sex adjusted age categories. Erythrocyte fatty acid composition was analyzed with a standardized methodology. The percentage of EPA and DHA in relation to all fatty acids present in the erythrocyte membrane is regarded the Omega-3 Index®.
Results
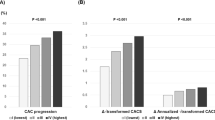
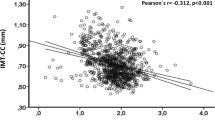
Among 71 patients, 51 were below and 20 were above the 75th AS-percentile. No differences were seen in age, gender, cardiovascular risk factors, and relevant medication. In univariable analysis, significantly lower values for EPA (0.77%[0.63; 0.97] vs. 0.93%[0.72; 1.21]; p = 0.045), DHA (4.90%[4.12; 5.57] vs. 5.50%[4.58; 6.52]; p = 0.038) and the Omega-3 Index (5.73%[4.75; 6.35] vs. 6.22%[5.46; 7.71]; p = 0.034) were seen in patients above the 75th AS-percentile. All other fatty acids showed no significant differences. In multivariable analysis, the Omega-3 Index showed a significant inverse association with early onset of CAC (OR: 0.533 (95%CI: 0.303–0.938; p = 0.029)), independent of age, gender, statin use, and creatinine level (all p > 0.05).
Conclusions
Low levels of EPA and DHA (Omega-3 Index) are associated with early-onset coronary atherosclerosis. This finding needs to be validated in larger cohorts and might help understand the beneficial cardiovascular effects of omega-3 fatty acids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Burr ML, Sweetham PM, Fehily AM. Diet and reinfarction. Eur Heart J. 1994;15:1152–3.
Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–55.
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8.
Harris WS, Tintle NL, Etherton MR, Vasan RS. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: The Framingham Heart Study. J Clin Lipido. 2018;12:718–27 e6.
Kleber ME, Delgado GE, Lorkowski S, Marz W, von Schacky C. Omega-3 fatty acids and mortality in patients referred for coronary angiography. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis. 2016;252:175–81.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2018.
Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res. 2006;98:727–9.
Schlemmer CK, Coetzer H, Claassen N, Kruger MC, Rademeyer C, van Jaarsveld L, et al. Ectopic calcification of rat aortas and kidneys is reduced with n-3 fatty acid supplementation. Prostaglandins Leukot Ess Fat Acids. 1998;59:221–7.
Bittner DO, Mayrhofer T, Bamberg F, Hallett TR, Janjua S, Addison D, et al. Impact of coronary calcification on clinical management in patients with acute chest pain. Circ Cardiovasc Imaging. 2017;10:e005893.
Bittner DO, Takx RAP, Staziaki PV, Janjua S, Neilan TG, Meyersohn NM, et al. Predictive value of coronary artery calcification and TIMI risk score in the assessment of patients with acute chest pain: results from an ED registry. J Am Coll Cardiol. 2016; 67:515.
Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700.
Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70.
Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39:2401–8.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20.
Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–7.
Niki T, Wakatsuki T, Yamaguchi K, Taketani Y, Oeduka H, Kusunose K, et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ J. 2016;80:450–60.
Nelson JR, Wani O, May HT, Budoff M. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vasc Pharmacol. 2017;91:1–9.
Sekikawa A, Mahajan H, Kadowaki S, Hisamatsu T, Miyagawa N, Fujiyoshi A, et al. Association of blood levels of marine omega-3 fatty acids with coronary calcification and calcium density in Japanese men. Eur J Clin Nutr. 2019;73:783–92.
Sekikawa A, Miura K, Lee S, Fujiyoshi A, Edmundowicz D, Kadowaki T, et al. Long chain n-3 polyunsaturated fatty acids and incidence rate of coronary artery calcification in Japanese men in Japan and white men in the USA: population based prospective cohort study. Heart 2014;100:569–73.
Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: Rationale and design of the EVAPORATE study. Clin Cardiol. 2018;41:13–9.
Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–82.
Harris WS, Kennedy KF, O'Keefe JH Jr., Spertus JA. Red blood cell fatty acid levels improve GRACE score prediction of 2-yr mortality in patients with myocardial infarction. Int J Cardiol. 2013;168:53–9.
Iwamatsu K, Abe S, Nishida H, Kageyama M, Nasuno T, Sakuma M, et al. Which has the stronger impact on coronary artery disease, eicosapentaenoic acid or docosahexaenoic acid? Hypertens Res. 2016;39:272–5.
von Schacky C. Omega-3 index and cardiovascular health. Nutrients. 2014;6:799–814.
Cawood AL, Ding R, Napper FL, Young RH, Williams JA, Ward MJ, et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–9.
Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013;2:e000513.
Köhler A, Bittner D, Löw A, von Schacky C. Effects of a convenience drink fortified with n-3 fatty acids on the n-3 index. Br J Nutr. 2010;104:729–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MM reports honoraria from Siemens HealthCare and Edwards Lifescience outside the submitted work, and YZ reports lecture fees from Baxter, MSD and Shire outside the submitted work. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bittner, D.O., Goeller, M., Zopf, Y. et al. Early-onset coronary atherosclerosis in patients with low levels of omega-3 fatty acids. Eur J Clin Nutr 74, 651–656 (2020). https://doi.org/10.1038/s41430-019-0551-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0551-5
This article is cited by
-
New Insights into Prospective Health Potential of ω-3 PUFAs
Current Nutrition Reports (2023)