Abstract
Objectives
Adequate vitamin B12 is a requisite during pregnancy and its deficiency is linked with increased risk for adverse outcomes, likely mediated by impaired placental angiogenesis. Thus, we aimed to test associations of maternal vitamin B12 status with the placental expression of angiogenesis-associated genes ENG, VEGF, and FLT.
Subjects/Methods
In this retrospective case-control study, placental and maternal trimester 1 blood samples (n = 104) were collected from small for gestational age (SGA) and appropriate for gestational age (AGA) full-term singleton pregnancies. Maternal trimester 1 vitamin B12 status was measured. Placentae and neonates were weighed at birth. Realtime quantitative PCR was performed to assess placental transcript abundance of ENG, VEGF, and FLT normalized to a panel of reference genes. Associations of placental transcript abundance of the genes with maternal trimester 1 vitamin B12 status were evaluated.
Results
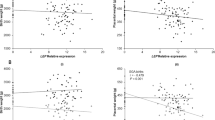
Placental ENG transcript abundance associated negatively with maternal trimester 1 vitamin B12 status (β = −0.461, P = 0.017, n = 104). This association was specific to the female births (β = −0.590, P = 0.014, n = 60). Placental VEGF transcript levels were negatively associated with maternal trimester 1 vitamin B12 status only in the female births (β = −1.995, P = 0.029). Placental FLT transcript levels were not associated with maternal trimester 1 vitamin B12 status.
Conclusion
Maternal trimester 1 vitamin B12 status was associated negatively with placental ENG and VEGF expression predominantly in the female births. Therefore, we hypothesize that the placenta adapts to low maternal vitamin B12 status by up-regulating angiogenic pathways in a gender-specific manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others

References
WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Tech Rep Ser. 1995;854:1–452.
Kramer M. Balanced protein/energy supplementation in pregnancy (Cochrane review). Cochrane Database Syst Rev. 2003;4:CD000032.
Guerra-Shinohara E, Morita O, Peres S, Pagliusi R, Sampaio Neto L, D’Almeida V, et al. Low ratio of S-adenosylmethionine to S-adenosylhomocysteine is associated with vitamin deficiency in Brazilian pregnant women and newborns. Am J Clin Nutr. 2004;80:1312–21.
Rogers L, Boy E, Miller J, Green R, Sabel J, Allen L. High prevalence of cobalamin deficiency in Guatemalan school children: associations with low plasma holotranscobalamin II and elevated serum methylmalonic acid and plasma homocysteine concentrations. Am J Clin Nutr. 2003;77:433–40.
Siekmann J, Allen L, Bwibo N, Demment M, Murphy S, Neumann C. Kenyan school children have multiple micronutrient deficiencies, but increased plasma vitamin B12 is the only detectable micronutrient response to meat or milk supplementation. J Nutr. 2003;133(11 Suppl 2):3972S–80S.
Bondevik GT, Schneede J, Refsum H, Lie RT, Ulstein M, Kvåle G. Homocysteine and methylmalonic acid levels in pregnant Nepali women. Should cobalamin supplementation be considered? Eur J Clin Nutr. 2001;55:856–64.
Allen L, Rosado J, Casterline J, López P, Muñoz E, Garcia O, et al. Lack of hemoglobin response to iron supplementation in anemic mexican preschoolers with multiple micronutrient deficiencies. Am J Clin Nutr. 2000;71:1485–94.
Refsum H, Yajnik C, Gadkari M, Schneede J, Vollset S, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–41.
Baik H, Russell R. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19:357–77.
Muthayya S, Kurpad A, Duggan C, Bosch R, Dwarkanath P, Mhaskar A, et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr. 2006;60:791–801.
Muthayya S, Dwarkanath P, Mhaskar M, Mhaskar R, Thomas A, Duggan C, et al. The relationship of neonatal serum vitamin B12 status with birthweight. Asia Pac J Clin Nutr. 2006;15:538–43.
Sukumar N, Rafnsson SB, Kandala N-B, Bhopal R, Yajnik CS, Saravanan P. Prevalence of vitamin B12 insufficiency during pregnancy and its effect on offspring birth weight: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103:1232–51.
Rogne T, Tielemans MJ, Chong MF, Yajnik CS, Krishnaveni GV, Poston L, et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: a systematic review and meta-analysis of individual participant data. Am J Epidemiol. 2017;185:212–23.
Maynard S, Epstein F, Karumanchi S. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78.
Plaisier M. Decidualisation and angiogenesis. Best Pr Res Clin Obs Gynaecol. 2011;25:259–71.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9.
Maynard SE, Min J-Y, Merchan J, Lim K-H, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58.
Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
Duggan C, Srinivasan K, Thomas T, Samuel T, Rajendran R, Muthayya S, et al. Vitamin B12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B12 status. J Nutr. 2014;144:758–64.
Mukhopadhyay A, Ravikumar G, Dwarkanath P, Meraaj H, Thomas A, Crasta J, et al. Placental expression of the insulin receptor binding protein GRB10: relation to human fetoplacental growth and fetal gender. Placenta. 2015;36:1225–30.
Mukhopadhyay A, Ravikumar G, Meraaj H, Dwarkanath P, Thomas A, Crasta J, et al. Placental expression of DNA methyltransferase 1 (DNMT1): gender-specific relation with human placental growth. Placenta. 2016;48:119–25.
Carroll MD, Curtin LR. Influential observations in the National Health and Nutrition Examination Survey, 1999–2000. In JSM Proceedings. Alexandria, VA: American Statistical Association 2002. p. 403--408.
Swanson DA, Liu M-L, Baker PJ, Garrett L, Stitzel M, Wu J, et al. Targeted disruption of the methionine synthase gene in mice. Mol Cell Biol. 2001;21:1058–65.
Hollegaard B, Byars SG, Lykke J, Boomsma JJ. Parent–offspring conflict and the persistence of pregnancy-induced hypertension in modern humans. Vitzthum VJ, editor. PLoS ONE. 2013;8:e56821.
Broere-Brown Z, Schalekamp-Timmermans S, Hofman A, Jaddoe V, Steegers E. Fetal sex dependency of maternal vascular adaptation to pregnancy: a prospective population-based cohort study. BJOG. 2016;123:1087–95.
Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci USA. 2006;103:5478–83.
Szentpéteri I, Rab A, Kornya L, Kovács P, Joó JG. Gene expression patterns of vascular endothelial growth factor (VEGF-A) in human placenta from pregnancies with intrauterine growth restriction. J Matern Fetal Neonatal Med. 2013;26:984–9.
Yinon Y, Nevo O, Xu J, Many A, Rolfo A, Todros T, et al. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-β3. Am J Pathol. 2008;172:77–85.
Milovanovic I, Njuieyon F, Deghmoun S, Chevenne D, Levy-Marchal C, Beltrand J. Innate small babies are metabolically healthy children. J Clin Endocrinol Metab. 2012;97:4407–13.
Acknowledgements
We thank the pregnant women who participated in the study and doctors and nurses who made this study possible. The contribution of the research assistants Ms. Nancy, Ms. Roopa, Ms. Aruna, and Ms. Mary who collected the samples and data is acknowledged. Histopath technician Ms. Mahalakshmi assisted the pathologists in placental grossing experiments. We gratefully acknowledge the guiding role of Prof. T.S. Sridhar in initial establishment of human placental tissue-related protocols.
Funding
This work was supported by the Department of Biotechnology, Government of India (Grant Sanction order BT/PR10632/PFN/20/838/2013 to AM, AVK, JC, and PD and grant sanction order 102/IFD/SAN/749/2014-2015 AM, AVK, and PD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mani, C., Kochhar, P., Ravikumar, G. et al. Placental expression of ENG, VEGF, and FLT: Gender-specific associations with maternal vitamin B12 status. Eur J Clin Nutr 74, 176–182 (2020). https://doi.org/10.1038/s41430-019-0449-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0449-2
This article is cited by
-
Placental expression of miR-21-5p, miR-210-3p and miR-141-3p: relation to human fetoplacental growth
European Journal of Clinical Nutrition (2022)