Abstract
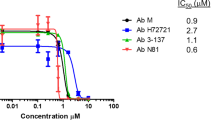
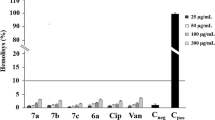
Antimicrobial resistance has emerged as a covert global health crisis, posing a significant threat to humanity. If left unaddressed, it is poised to become the foremost cause of mortality worldwide. Among the multitude of resistant bacterial pathogens, Pseudomonas aeruginosa, a Gram-negative, facultative bacterium, has been responsible for mild to deadly infections. It is now enlisted as a global critical priority pathogen by WHO. Urgent measures are required to combat this formidable pathogen, necessitating the development of novel anti-pseudomonal drugs. To confront this pressing issue, we conducted an extensive screening of 3561 compounds from the ChemDiv library, resulting in the discovery of potent anti-pseudomonal quinazoline derivatives. Among the identified compounds, IDD-8E has emerged as a lead molecule, exhibiting exceptional efficacy against P. aeruginosa while displaying no cytotoxicity. Moreover, IDD-8E demonstrated significant pseudomonal killing, disruption of pseudomonal biofilm and other anti-bacterial properties comparable to a well-known antibiotic rifampicin. Additionally, IDD-8E’s synergy with different antibiotics further strengthens its potential as a powerful anti-pseudomonal agent. IDD-8E also exhibited significant antimicrobial efficacy against other ESKAPE pathogens. Moreover, we elucidated the Structure-Activity-Relationship (SAR) of IDD-8E targeting the essential WaaP protein in P. aeruginosa. Altogether, our findings emphasize the promise of IDD-8E as a clinical candidate for novel anti-pseudomonal drugs, offering hope in the battle against antibiotic resistance and its devastating impact on global health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022;20:193–205.
Bloom DE, Cadarette D. Infectious Disease threats in the twenty-first century: strengthening the global response. Front Immunol. 2019;10:549.
Piret J, Boivin G. Pandemics throughout history. Front Microbiol. 2020;11:631736.
Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–83.
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58.
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109:309–18.
Larkin H. Increasing antimicrobial resistance poses global threat, WHO says. JAMA 2023;329:200.
Cameron A, Esiovwa R, Connolly J, Hursthouse A, Henriquez F. Antimicrobial resistance as a global health threat: the need to learn lessons from the COVID-19 pandemic. Glob Policy. 2022;13:179–92.
EclinicalMedicine. Antimicrobial resistance: a top ten global public health threat. EClinicalMedicine. 2021;41:101221.
de Kraker ME, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13:e1002184.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55.
Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10:841–51.
Jurado-Martin I, Sainz-Mejias M, McClean S. Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int J Mol Sci. 2021;22:3128.
Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7:199.
Wood SJ, Kuzel TM, Shafikhani SH. Pseudomonas aeruginosa: infections, animal modeling, and therapeutics. Cells. 2023;12:199.
Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–73.
Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–60.
Yin R, Cheng J, Wang J, Li P, Lin J. Treatment of Pseudomonas aeruginosa infectious biofilms: challenges and strategies. Front Microbiol. 2022;13:955286.
Davies O, Bennett S. WHO publishes list of bacteria for which new antibiotics are urgently needed. WHO Newsletters. 2017.
Jayaraman N, Mahapa A, Samanta G, Maiti K, Chatterji D. Mannopyranoside glycolipids inhibit mycobacterial growth, biofilm and potentiate isoniazid inhibition activities in M. smegmatis. ChemBioChem. 2019;20:1966–76.
Dolan SK. Current knowledge and future directions in developing strategies to combat Pseudomonas aeruginosa Infection. J Mol Biol. 2020;432:5509–28.
Verderosa AD, Totsika M, Fairfull-Smith KE. Bacterial biofilm eradication agents: a current review. Front Chem. 2019;7:824.
Jangra V, Sharma N, Chhillar AK. Therapeutic approaches for combating Pseudomonas aeruginosa infections. Microbes Infect. 2022;24:104950.
Chatterjee M, Anju CP, Biswas L, Anil Kumar V, Gopi Mohan C, Biswas R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol. 2016;306:48–58.
Schnappinger D. Genetic approaches to facilitate antibacterial drug development. Cold Spring Harb Perspect Med. 2015;5:a021139.
Matano LM, Morris HG, Wood BM, Meredith TC, Walker S. Accelerating the discovery of antibacterial compounds using pathway-directed whole cell screening. Bioorg Med Chem. 2016;24:6307–14.
Brotz-Oesterhelt H, Sass P. Postgenomic strategies in antibacterial drug discovery. Future Microbiol. 2010;5:1553–79.
Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–19.
Lechartier B, Rybniker J, Zumla A, Cole ST. Tuberculosis drug discovery in the post-post-genomic era. EMBO Mol Med. 2014;6:158–68.
Miethke M, Pieroni M, Weber T, Bronstrup M, Hammann P, Halby L, et al. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem. 2021;5:726–49.
Boyd NK, Teng C, Frei CR. Brief overview of approaches and challenges in new antibiotic development: a focus on drug repurposing. Front Cell Infect Microbiol. 2021;11:684515.
Zhao X, Lam JS. WaaP of Pseudomonas aeruginosa is a novel eukaryotic type protein-tyrosine kinase as well as a sugar kinase essential for the biosynthesis of core lipopolysaccharide. J Biol Chem. 2002;277:4722–30.
Sarkar B, Mahapa A, Dey K, Manhas R, Chatterji D, Jayaraman N. Aza-Michael promoted glycoconjugation of PETIM dendrimers and selectivity in mycobacterial growth inhibitions. RSC Adv. 2023;13:4669–77.
Nadri MH, Salim Y, Basar N, Yahya A, Zulkifli RM. Antioxidant activities and tyrosinase inhibition effects of Phaleria macrocarpa extracts. Afr J Tradit Complement Alter Med. 2014;11:107–11.
Clinical, Institute LS. Performance standards for antimicrobial susceptibility testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. p. 106–12.
Mahapa A, Samanta GC, Maiti K, Chatterji D, Jayaraman N. Mannopyranoside glycolipids inhibit mycobacterial and biofilm growth and potentiate isoniazid inhibition activities in M. smegmatis. ChemBioChem. 2019;20:1966–76.
Lambert RJ, Pearson J. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J Appl Microbiol. 2000;88:784–90.
Van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–45.
Standards NCfCL, Barry AL. Methods for determining bactericidal activity of antimicrobial agents: approved guideline. Wayne, PA: National Committee for Clinical Laboratory Standards; 1999.
Maiti K, Syal K, Chatterji D, Jayaraman N. Synthetic arabinomannan heptasaccharide glycolipids inhibit biofilm growth and augment isoniazid effects in mycobacterium smegmatis. ChemBioChem. 2017;18:1959–70.
Caiazza NC, Shanks RM, O’toole G. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol. 2005;187:7351–61.
Bellio P, Fagnani L, Nazzicone L, Celenza G. New and simplified method for drug combination studies by checkerboard assay. MethodsX. 2021;8:101543.
Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol. 2005;43:140–3.
Hall M, Middleton R, Westmacott D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J Antimicrob Chemother. 1983;11:427–33.
Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1.
Kreamer NNK, Chopra R, Caughlan RE, Fabbro D, Fang E, Gee P, et al. Acylated-acyl carrier protein stabilizes the Pseudomonas aeruginosa WaaP lipopolysaccharide heptose kinase. Sci Rep. 2018;8:14124.
Gautam S, Mahapa A, Yeramala L, Gandhi A, Krishnan S, Kutti RV, et al. Regulatory mechanisms of c‐di‐AMP synthase from Mycobacterium smegmatis revealed by a structure: function analysis. Protein Sci. 2023;32:e4568.
Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–4.
Șandor A, Ionuț I, Marc G, Oniga I, Eniu D, Oniga O. Structure–activity relationship studies based on quinazoline derivatives as EGFR kinase inhibitors (2017–Present). Pharmaceuticals. 2023;16:534.
Meletiadis J, Pournaras S, Roilides E, Walsh TJ. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob Agents Chemother. 2010;54:602–9.
Karan R, Agarwal P, Sinha M, Mahato N. Recent advances on quinazoline derivatives: a potential bioactive scaffold in medicinal chemistry. ChemEngineering. 2021;5:73.
Shang XF, Morris-Natschke SL, Yang GZ, Liu YQ, Guo X, Xu XS, et al. Biologically active quinoline and quinazoline alkaloids part II. Med Res Rev. 2018;38:1614–60.
Shang XF, Morris-Natschke SL, Liu YQ, Guo X, Xu XS, Goto M, et al. Biologically active quinoline and quinazoline alkaloids part I. Med Res Rev. 2018;38:775–828.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl J Med. 2004;350:2129–39.
Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib) relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–9.
Raymond E, Faivre S, Armand JP. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs 2000;60:15–23.
Dungo RT, Keating GM. Afatinib: first global approval. Drugs. 2013;73:1503–15.
Shao H, Wells A. Deciphering the molecular mechanism of enhanced tumor activity of the EGFR variant T790M/L858R using melanoma cell lines. Front Oncol. 2023;13:1163504.
Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81.
Guo Y, Du Z, Shi T. Structural Analysis of Interactions between Epidermal Growth Factor Receptor (EGFR) Mutants and Their Inhibitors. Biophysica. 2023;3:203–13.
Amelia T, Kartasasmita RE, Ohwada T, Tjahjono DH. Structural insight and development of EGFR tyrosine kinase inhibitors. Molecules. 2022;27:819.
Yethon JA, Whitfield C. Purification and characterization of WaaP from Escherichia coli, a lipopolysaccharide kinase essential for outer membrane stability. J Biol Chem. 2001;276:5498–504.
Lam JS, Taylor VL, Islam ST, Hao Y, Kocincova D. Genetic and functional diversity of Pseudomonas aeruginosa lipopolysaccharide. Front Microbiol. 2011;2:118.
Walsh AG, Matewish MJ, Burrows LL, Monteiro MA, Perry MB, Lam JS. Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Mol Microbiol. 2000;35:718–27.
Le Y, Gan Y, Fu Y, Liu J, Li W, Zou X, et al. Design, synthesis and in vitro biological evaluation of quinazolinone derivatives as EGFR inhibitors for antitumor treatment. J Enzym Inhib Med Chem. 2020;35:555–64.
Jafari E, Khajouei MR, Hassanzadeh F, Hakimelahi GH, Khodarahmi GA. Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res Pharm Sci. 2016;11:1–14.
Jadhavar PS, Patel KI, Dhameliya TM, Saha N, Vaja MD, Krishna VS, et al. Benzimidazoquinazolines as new potent anti-TB chemotypes: Design, synthesis, and biological evaluation. Bioorg Chem. 2020;99:103774.
Gawad J, Bonde C. Design, synthesis and biological evaluation of novel 6-(trifluoromethyl)-N-(4-oxothiazolidin-3-yl) quinazoline-2-carboxamide derivatives as a potential DprE1 inhibitors. J Mol Struct. 2020;1217:128394.
Chevalier J, Mahamoud A, Baitiche M, Adam E, Viveiros M, Smarandache A, et al. Quinazoline derivatives are efficient chemosensitizers of antibiotic activity in Enterobacter aerogenes, Klebsiella pneumoniae and Pseudomonas aeruginosa resistant strains. Int J Antimicrob Agents. 2010;36:164–8.
Deka B, Suri M, Sarma S, Devi MV, Bora A, Sen T, et al. Potentiating the intracellular killing of Staphylococcus aureus by dihydroquinazoline analogues as NorA efflux pump inhibitor. Bioorg Med Chem. 2022;54:116580.
Cedraro N, Cannalire R, Astolfi A, Mangiaterra G, Felicetti T, Vaiasicca S, et al. From Quinoline to Quinazoline-Based S. aureus NorA Efflux Pump Inhibitors by Coupling a Focused Scaffold Hopping Approach and a Pharmacophore Search. ChemMedChem. 2021;16:3044–59.
Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci. 2004;101:13306–11.
Voigtlaender M, Schneider-Merck T, Trepel M. Lapatinib. Small Mol Oncol. 2018;211:19–44.
Guillon RM, Pagniez F, Picot C, Hédou D, Tonnerre A, Chosson E, et al. Discovery of a novel broad-spectrum antifungal agent derived from albaconazole. ACS Med Chem Lett. 2013;4:288–92.
Pasqualotto A, Denning D. New and emerging treatments for fungal infections. J Antimicrob Chemother. 2008;61:i19–i30.
Santos-Ballardo L, Garcia-Paez F, Picos-Corrales LA, Ochoa-Teran A, Bastidas P, Calderón-Zamora L, et al. Synthesis, biological evaluation and molecular docking of 3-substituted quinazoline-2, 4 (1 H, 3 H)-diones. J Chem Sci. 2020;132:1–10.
Kashaw SK, Kashaw V, Mishra VK, Mishra M, Agrawal RK. Novel Quinazoline-Chalcone Hybrids As Antiplasmodium Agents: Synthesis, Biological Evaluation And Molecular Docking. J Adv Sci Res. 2020;11:85–99.
El Helou G, Razonable RR. Letermovir for the prevention of cytomegalovirus infection and disease in transplant recipients: an evidence-based review. Infect Drug Resist. 2019;12:1481–91.
Wang M, Zhang G, Wang Y, Wang J, Zhu M, Cen S, et al. Design, synthesis and anti-influenza A virus activity of novel 2, 4-disubstituted quinazoline derivatives. Bioorg Med Chem Lett. 2020;30:127143.
Zhang GP, Pan JK, Zhang J, Wu ZX, Liu DY, Zhao L. Design, synthesis, antiviral activities of novel phosphonate derivatives containing quinazoline based on chalone motif. J Heterocycl Chem. 2017;54:2548–55.
Janardhanan J, Bouley R, Martinez-Caballero S, Peng Z, Batuecas-Mordillo M, Meisel JE, et al. The quinazolinone allosteric inhibitor of PBP 2a synergizes with piperacillin and tazobactam against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2019;63:e02637–18.
Kumar AS, Kudva J, Lahtinen M, Peuronen A, Sadashiva R, Naral D. Synthesis, characterization, crystal structures and biological screening of 4-amino quinazoline sulfonamide derivatives. J Mol Struct. 2019;1190:29–36.
Acknowledgements
The authors are thankful to CSIR-IIIM (MLP-21005) and Science and Engineering Research Board- Start-up Research Grant (SERB-SRG) Grant no: SRG/2023/000145 for funding. AR is thankful for the UGC JRF fellowship. The authors acknowledge Jyoti Kumari and Sapna Rajput for their assistance in time kill assay (CFU method) and ChemDiv compound dilutions, respectively. This manuscript has been given institutional number: CSIR-IIIM/IPR/00633, dated:10/20/2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manhas, R., Rathore, A., Havelikar, U. et al. Uncovering the potentiality of quinazoline derivatives against Pseudomonas aeruginosa with antimicrobial synergy and SAR analysis. J Antibiot 77, 365–381 (2024). https://doi.org/10.1038/s41429-024-00717-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-024-00717-3