Abstract
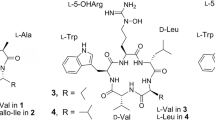
Tripropeptin C, a non-ribosomal cyclic lipopeptide containing three proline residues, exhibits excellent efficacy in the mouse-methicillin-resistant Staphylococcus aureus septicemia model. Since tripropeptins contain L-prolyl-D-proline and, as a result, are known to form a hairpin structure in proteins, it was of interest to determine whether this substructure contributes to their antibacterial activity. In this study, prolines in tripropeptin C were replaced with pipecolic acid(s) using precursor-directed biosynthesis. Only a new tripropeptin analog, tripropeptin Cpip, which has one L-pipecolic acid in place of L-proline, was isolated. The in vitro antimicrobial activity of the new analog was approximately two to four times weaker activity against Gram-positive bacteria, including drug-resistant species, compared with that of tripropeptin C. These results suggest that the L-prolyl-D-proline substructure plays an important role in the observed potency of tripropeptins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hashizume H, et al. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. I. Taxonomy, isolation, and biological activities. J Antibiot. 2001;54:1054–9.
Hashizume H, et al. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. II. Structure elucidation. J Antibiot. 2004;57:52–8.
Hashizume H, Hattori S, Igarashi M, Akamatsu Y. Tripropeptin E, a new tripropeptin group antibiotic produced by Lysobacter sp. BMK333-48F4. J Antibiot. 2004;57:394–9.
Hashizume H, et al. Tripropeptin C blocks the lipid cycle of cell wall biosynthesis by complex formation with undecaprenyl pyrophosphate. Antimicrob Agents Chemother. 2011;55:3821–8.
Hirosawa S, Takahashi Y, Hashizume H, Miyake T, Akamatsu Y. Synthesis and antibacterial activity of tripropeptin C derivatives modified at the carboxyl groups. J Antibiot. 2014;67:265–8.
Hashizume H, Takahashi Y, Harada S, Nomoto A. Natural lipopeptide antibiotic tripropeptin C revitalizes and synergistically potentiates the activity of beta-lactam against methicillin-resistant Staphylococcus aureus. J Antibiot. 2015;68:373–8.
Hashizume H, et al. In vivo efficacy of β-lactam/tripropeptin C in a mouse septicemia model and the mechanism of reverse β-lactam resistance in methicillin-resistant Staphylococcus aureus mediated by tripropeptin C. J Antibiot. 2018;71:79–85.
Hirosawa S, et al. Synthesis and antibacterial activity of tripropeptin C derivatives containing the pyrimidine system. Heterocycles. 2015;91:2143–51.
Hirosawa S, et al. In vitro antimicrobial activity and in vivo efficacy in a mouse staphylococcal-septicemia model of water-soluble tripropeptin C analogs [abstract F1-1509]. In: 52nd ICAAC. American Society for Microbiology (ASM): 2012.
Hashizume H, et al. Production of tripropeptins in media supplemented with precursors based on the biosynthetic pathway. ARKIVOC. 2007;7:241–53.
Hashizume H, et al. A new type of tripropeptin with anteiso-branched chain fatty acid from Lysobacter sp. BMK333-48F3. J Antibiot. 2008;61:577–82.
Harada K, et al. Application of D, L-FDLA derivatization to determination of absolute configuration of constituent amino acids in peptide by advanced Marfey’s method. Tetrahedron Lett. 1996;37:3001–4.
Sawa R, et al. Amycolamicin: a novel broad-spectrum antibiotic inhibiting bacterial topoisomerase. Chem Eur J. 2012;18:15772–81.
Konishi M, et al. Empedopeptin (BMY-28117), a new depsipeptide antibiotic I. production, isolation and properties. J Antibiot. 1984;37:949–57.
Shoji J, et al. Isolation and characterization of new peptide antibiotics, plusbacins A1-A4 and B1-B4. J Antibiot. 1992;45:817–23.
Muller A, et al. Lipodepsipeptide empedopeptin inhibits cell wall biosynthesis through Ca2+-dependent complex formation with peptidoglycan precursors. J Biol Chem. 2012;287:20270–80.
Bean JW, Kopple KD, Peishoff CE. Conformational analysis of cyclic hexapeptides containing the D-Pro-L-Pro sequence to fix beta-turn positions. J Am Chem Soc. 1992;114:5328–34.
Kino T, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. fermentation, isolation and physico-chemical and biological characteristics. J Antibiot. 1987;40:1249–55.
Wong GK, Griffith S, Kojima I, Demain AL. Antifungal activities of rapamycin and its derivatives, prolylrapamycin, 32-desmethylrapamycin, and 32-desmethoxyrapamycin. J Antibiot. 1998;51:487–91.
Son S, et al. A pipecolic acid-rich branched cyclic depsipeptide ulleungamide C from a Streptomyces species induced G0/G1 cell cycle arrest in promyelocytic leukemia cells. J Antibiot. 2021;74:181–9.
Acknowledgements
The authors express our special thanks to Mr. Kunio Inoue for the measurement of MICs and to Dr. Tomoyuki Kimura for his valuable discussion. This work was supported by the Japan Society for the Promotion of Science KAKENHI, grant numbers 24710254 and 16K08338. We thank Dr. Renee Mosi, and Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashizume, H., Sawa, R., Kubota, Y. et al. Precursor-directed biosynthesis and biological activity of tripropeptin Cpip, a new tripropeptin C analog containing pipecolic acid. J Antibiot 77, 238–244 (2024). https://doi.org/10.1038/s41429-024-00703-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-024-00703-9