Abstract
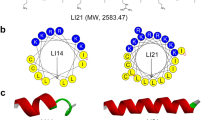
As the important components of biological innate immunity, antimicrobial peptides (AMPs) were found in a variety of organisms including insects, plants, animals, bacteria, fungi, etc. However, high hemolytic activity, high toxicity, and poor stability of natural AMPs hinder serious their application as therapeutic agents. To overcome these problems, in this study we use PRRP as a central axis, and peptides were designed based on the sequence template XRRXXRXPRRPXRXXRRX-NH2, where X represents a hydrophobic amino acid like Phe (F), Ile (I), Val (V), and Leu (L). The designed peptides LR18, FR18, and IR18 showed effective antimicrobial activity against some Gram-positive bacteria and Gram-negative bacteria, low cytotoxicity to mammalian cells, and had a tendency to form α-helical structures in membrane-mimetic environments. Among them, peptide LR18 (X: L) showed the highest geometric mean average treatment index (GMTI = 42.7) against Gram-negative bacteria, and FR18 (X: L) showed the highest GMTI (22.86) against Gram-positive bacteria. LR18 and FR18 also showed better salt, temperature, pH, and trypsin stability. LR18 and FR18 exert their antimicrobial effects mainly through destroying bacteria cell membrane. Briefly, peptide LR18 and FR18 have the potential to serve as a therapeutic agent to reduce antibiotic resistance owing to its high therapeutic index and great stability.
Highlights
-
Introducing symmetry axis PRRP to the sequence of the typical α-helical antimicrobial peptides can improve their therapeutic index and stability.
-
The designed peptides LR18, FR18, and IR18 all have broad-spectrum and efficient antibacterial activity.
-
LR18 showed the highest average therapeutic index (GMTI = 42.7) against Gram-negative bacteria, and FR18 showed the highest (GMTI = 22.86) against Gram-positive bacteria.
-
Both the designed peptides LR18 and FR18 showed good thermal stability, pH stability, and physiological salt stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Tiseo K, Huber L, Gilbert M, Robinson TP, Van Boeckel TP. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. 2015;112:5649–54.
Pei Z, et al. Biological characteristics of a new antibacterial peptide and its antibacterial mechanisms against Gram-negative bacteria. Polish J Vet Sci. 2018;21:533–42.
Lee W, Lee DG. Fungicidal mechanisms of the antimicrobial peptide Bac8c. Biochim Biophys Acta (BBA) Biomembr. 2015;1848:673–9.
Wang J, et al. Antimicrobial peptides: promising alternatives in the postfeeding antibiotic era. Med Res Rev. 2019;39:831–59.
Ahmad A, et al. Design of nontoxic analogues of cathelicidin-derived bovine antimicrobial peptide BMAP-27: the role of leucine as well as phenylalanine zipper sequences in determining its toxicity. Biochemistry. 2009;48:10905–17.
Kumar A, et al. Single amino acid substitutions at specific positions of the heptad repeat sequence of piscidin-1 yielded novel analogs that show low cytotoxicity and in vitro and in vivo antiendotoxin activity. Antimicrobial Agents Chemother. 2016;60:3687–99.
Jia B, et al. High cell selectivity and bactericidal mechanism of symmetric peptides centered on d-Pro–Gly pairs. Int J Mol Sci. 2020;21:1140.
Wang J, et al. Combating drug-resistant fungi with novel imperfectly amphipathic palindromic peptides. J Med Chem. 2018;61:3889–907.
Shao C, et al. Symmetrical modification of minimized dermaseptins to extend the spectrum of antimicrobials with endotoxin neutralization potency. Int J Mol Sci. 2019;20:1417.
Zhang Y, et al. iTRAQ-based quantitative proteomics analysis reveals inhibitory mechanisms of the antimicrobial peptide MDAP-2 against Salmonella gallinarum. Pol J Vet Sci. 2020;23:405–14.
Huang Y, Huang J, Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell. 2010;1:143–52.
Chen Y, et al. Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/ therapeutic index. J Biol Chem. 2005;280:12316–29.
Zhu X, et al. Design of imperfectly amphipathic α-helical antimicrobial peptides with enhanced cell selectivity. Acta Biomater. 2014;10:244–57.
Lee J, Lee DG. Antimicrobial peptides (AMPs) with dual mechanisms: membrane disruption and apoptosis. J Microbiol Biotechnol. 2015;25:759–64.
Sass V, et al. Humanbeta-defensin3 inhibits cell wall biosynthesis in Staphylococci. Infect Immun. 2010;78:2793–2800.
Mardirossian M, et al. The host antimicrobial peptide Bac7 (1–35) binds to bacterial ribosomal proteins and inhibits protein synthesis. ChemBiol. 2014;21:1639–47.
Schmidtchen A, Pasupuleti M, Malmsten M. Effect of hydrophobic modifications in antimicrobial peptides. Adv Colloid Interface Sci. 2014;205:265–74.
Acknowledgements
This research was funded by the Natural Science Foundation of Jilin Province (Grant number: 20210101040JC), Science and technology project of the 13th five-year plan of Jilin Province Department of Education (Grant number: JJKH20200354KJ).
Author information
Authors and Affiliations
Contributions
ZP and HM conceived and designed the experiments; SY, YZ, and BJ performed the experiments; ZP and BJ analyzed the data; ZP and SY wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, S., Jia, B., Zhang, Y. et al. Design, biological characteristics, and antibacterial mechanism of high therapeutic index antimicrobial peptides with PRRP as central axis. J Antibiot 77, 170–181 (2024). https://doi.org/10.1038/s41429-023-00697-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00697-w