Abstract
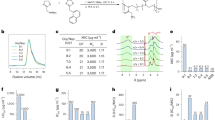
A new antifungal compound, named N-demethyltyroscherin (1), was discovered from the static fungal cultured material of Scedosporium apiospermum FKJ-0499 isolated from a deep-sea sediment sample together with a known compound, tyroscherin (2). The structure of 1 was elucidated as a new analog of 2 by MS and NMR analyses. The absolute configuration of 1 was determined by chemical derivatization. Both compounds showed potent in vitro antifungal activity against clinically isolated Candida auris strains, with MIC values ranging from 0.0625 to 4 µg ml–1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53:41–4.
Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–40.
Sanyaolu A, Okorie C, Marinkovic A, Abbasi AF, Prakash S, Mangat J, et al. Candida auris: An overview of the emerging drug-resistant fungal infection. Infect Chemother. 2022;54:236–46.
Watanabe Y, Yoshida Y, Tokiwa T, Higo M, Ban S, Ikeda A, et al. Hakuhybotric acid, a new antifungal polyketide produced by a mycoparasitic fungus Hypomyces pseudocorticiicola FKI-9008. J Gen Appl Microbiol. 2022;68:200–6.
Watanabe Y, Takahashi S, Ito S, Tokiwa T, Noguchi Y, Azami H, et al. Hakuhybotrol, a polyketide produced by Hypomyces pseudocorticiicola, characterized with the assistance of 3D ED/MicroED. Org Biomol Chem. 2023;21:2320–30.
Watanabe Y, Suga T, Narusawa S, Iwatsuki M, Nonaka K, Nakashima T, et al. Decatamariic acid, a new mitochondrial respiration inhibitor discovered by pesticidal screening using drug-sensitive Saccharomyces cerevisiae. J Antibiot. 2017;70:395–9.
Watanabe Y, Asami Y, Narusawa S, Hashimoto S, Iwatsuki M, Nonaka K, et al. Ascosteroside D, a new mitochondrial respiration inhibitor discovered by pesticidal screening using insect ADP/ATP carrier protein-expressing Saccharomyces cerevisiae. J Antibiot. 2018;71:146–8.
Hayakawa Y, Yamashita T, Mori T, Nagai K, Shin-ya K, Watanabe H. Structure of tyroscherin, an antitumor antibiotic against IGF-1-dependent cells from Pseudallescheria sp. J Antibiot. 2004;57:634–8.
Takahashi K, Sakai K, Nagano Y, Orui-Sakaguchi S, Lima AO, Pellizari VH, et al. Cladomarine, a new anti-saprolegniasis isolated from the deep-sea fungus, Penicillium coralligerum YK-247. J Antibiot. 2017;70:911–4.
Nonaka K, Ōmura S, Masuma R, Kaifuchi S, Masuma R. Three new Pochonia taxa (Clavicipitaceae) from soils in Japan. Mycologia. 2013;105:1202–18.
Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, et al. Papuamides A−D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J Am Chem Soc. 1999;121:5899–909.
Alexander BD, Procop GW, Dufresne P, Espinel-Ingroff A, Fuller J, Ghannoum MA, et al. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, M38. 3rd ed.: Clinical and Laboratory Standards Institute. 2017.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–402.
Gilgado F, Cano J, Gené J, Guarro J. Molecular phylogeny of the Pseudallescheria boydii species complex: Proposal of two new species. J Clin Microbiol. 2005;43:4930–42.
Guarro J, Kantarcioglu AS, Horré R, Rodriguez-Tudela JL, Estrella MC, Berenguer J, et al. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol. 2006;44:295–327.
Lackner M, Sybren de Hoog G, Yang L, Moreno LF, Ahmed SA, Andreas F, et al. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Diversity. 2014;67:1–10.
Vincenzo C, Johannes G, Johannes M. Antibiotic and pharmaceutical compositions containing it. DE1983-3333553.
CDC & FDA Antibiotic Resistance Isolate Bank; https://wwwn.cdc.gov/ARIsolateBank/Panel/PanelDetail?ID=2.
Nirma C, Eparvier V, Stien D. Antifungal agents from Pseudallescheria boydii SNB-CN73 isolated from a Nasutitermes sp. termite. J Nat Prod. 2013;76:988–91.
Kamigiri K, Tanaka K, Matsumoto H, Nagai K, Watanabe M, Suzuki K. YM-193221, a novel antifungal antibiotic produced by Pseudallescheria ellipsoidea. J Antibiot. 2004;57:569–72.
LaMonte G, Lim MY-X, Wree M, Reimer C, Nachon M, Corey V, et al. Mutations in the Plasmodium falciparum cyclic amine resistance locus (PfCARL) confer multidrug resistance. Mbio. 2016;7:e00696–16.
Sorres J, Nirma C, Barthélemy M, Eparvier V, Stien D. Tyroscherin and tyroscherin analogs from Pseudallescheria boydii SNB-CN85 isolated from termite Termes cf. hispaniolae. Phytochem Lett. 2017;22:142–4.
Chen R, Li Z, Qin C, Lu P, Lin J, Zheng W, et al. A novel antibacterial tyroscherin derivative with a natural unprecedented morpholine-2, 3-dione structural unit from the fungus Pseudallescheria boydii. Nat Prod Res. 2022;36:5977–83.
Katsuta R, Shibata C, Ishigami K, Watanabe H, Kitahara T. Synthesis and structure revision of tyroscherin, a growth inhibitor of IGF-1-dependent tumor cells. Tetrahedron Lett. 2008;49:7042–5.
Tae HS, Hines J, Schneekloth AR, Crews CM. Total synthesis and biological evaluation of tyroscherin. Org Lett. 2010;12:4308–11.
Ugele M, Maier ME. Synthesis of the alkaloid tyroscherin by an aldol/Curtius strategy. Tetrahedron. 2010;66:2633–41.
Yoon D-H, Ji M-K, Ha H-J, Park J, Kang P, Lee WK. Synthesis and biological activities of tyroscherin analogs. Bull Korean Chem Soc. 2013;34:1899–902.
Acknowledgements
We are grateful to Distinguished Emeritus Professor Satoshi Ōmura (Kitasato University) for his helpful support and valuable guidance and suggestions. We thank Dr. Kenichiro Nagai, Ms. Reiko Seki, and Ms. Noriko Sato (School of Pharmacy, Kitasato University) for various instrumental analyses. We also thank Dr. Hidetaka Nomaki for his great help in collecting the deep-sea sample. The authors would like to thank the Centers for Disease Control and Prevention and the National Institute of Infectious Diseases for providing the AR Isolate bank strains. This research was partially supported by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP21am0101096 (Phase I) and JP22ama121035 (Phase II). This work was supported by the JSPS KAKENHI Grant (21K05293 and 23H04887).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azami, H., Watanabe, Y., Sakai, K. et al. Antifungal profile against Candida auris clinical isolates of tyroscherin and its new analog produced by the deep-sea-derived fungal strain Scedosporium apiospermum FKJ-0499. J Antibiot 77, 156–162 (2024). https://doi.org/10.1038/s41429-023-00696-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00696-x
This article is cited by
-
Crowdsourcing for mining new fungal sources for addressing the need for novel antibiotics against multidrug resistant pathogens
The Journal of Antibiotics (2024)