Abstract
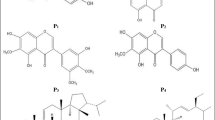
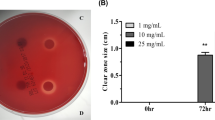
A previous study by our group demonstrated that a vitamin D3 decomposition product (VDP1) acts as the selective bactericidal substance on Helicobacter pylori. VDP1 is an indene compound modified with a carbonyl and an alkyl. The alkyl of VDP1 turned out to be a mandatory structure to exert effective bactericidal action on H. pylori. Meanwhile, it still remains to be clarified as to how influence the alteration of the carbonyl in VDP1 has on the anti-H. pylori activity. In this study, we synthesized novel VDP1 derivatives that replaced the carbonyl of VDP1 by various functional groups and investigated the antibacterial action of the VDP1 derivatives on H. pylori. VDP1 derivatives retaining either a hydroxy (VD3-1) or an acetic ester (VD3-3) exhibited more effective bactericidal action to H. pylori than VDP1. The replacement of the carbonyl of VDP1 by either an allyl acetate (VD3-2) or an acrylic acid (VD3-5) provided almost no change to the anti-H. pylori activity. Apart from this, an isomer of VDP1 (VD3-4) slightly improved anti-H. pylori activity of VDP1. Meanwhile, the replacement of the carbonyl of VDP1 by a methyl acrylate (VD3-6) attenuated the anti-H. pylori activity. As with VDP1, its derivatives also were suggested to exert the anti-H. pylori action through the interaction with myristic acid side chains of dimyristoyl-phosphatidylethanolamine, a characteristic membrane lipid constituent of this pathogen. These results indicate that it is capable of developing specific antibacterial medicines for H. pylori targeting the biomembranal dimyristoyl-phosphatidylethanolamine using VDP1 as the fundamental structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Marshall B, Warren JR. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet .1983;1:1273–4.
Graham DY. Helicobacter pylori: Its epidemiology and its role in duodenal ulcer disease. J Gastroenterol Hep. 1991;6:105–13.
Forman D. The Eurogast study group. An international association between Helicobacter pylori infection and gastric cancer. Lancet .1993;341:1359–63.
Wotherspoon AC, Oriz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet .1999;338:1175–6.
Uemura N, Okamoto S, Yamamoto S, Matsumura M, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. 2N Engl J Med. 2001;345:829–32.
Peek JRM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinoma. Nat Rev Cancer. 2002;2:28–37.
Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A. Helicobacter and gastric MALT lymphoma. Gut .2002;50:III19–24.
Moss SF. The clinical evidence linking Helicobacter pylori to gastric cancer. Cell Mol Gastroenterol Hepatol. 2017;3:183–91.
Francesco VD, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointest Liv Dis 2010;19:409–14.
Trifan A, Girleanu I, Cojocariu C, Sfarti C, Singeap AM, Dorobat C, Grigore L, Stanciu C. Pseudomembranous colitis associated with a triple therapy for Helicobacter pylori eradication. World J Gastroenterol. 2013;19:7476–9.
Hosoda K, Shimomura H, Wanibuchi K, Masui H, Amgalanbaatar A, Hayashi S, Takahashi T, Hirai Y. Identification and characterization of a vitamin D3 decomposition product bactericidal against Helicobacter pylori. Sci Rep. 2015;5:8860. https://doi.org/10.1038/srep08860.
Wanibuchi K, Hosoda K, Ihara M, Tajiri K, Sakai Y, Masui H, Takahashi T, Hirai Y, Shimomura H. Indene compounds synthetically derived from vitamin D have selective antibacterial action on Helicobacter pylori. Lipids .2018;53:393–401.
Wanibuchi K, Takezawa M, Hosoda K, Amgalanbaatar A, Tajiri K, Koizumi Y, Niitsu S, Masui H, Sakai Y, Shoji M, Takahashi T, Hirai Y, Shimomura H. Antibacterial effect of indene on Helicobacter pylori correlates with specific interaction between its compound and dimyristoyl-phosphatidylethanolamine. Chem. Phys. Lipids. 2020. https://doi.org/10.1016/j.chemphyslip.2020.104871.
Shimomura H, Wanibuchi K, Hosoda K, Amgalanbaatar A, Masui H, Takahashi T, Hirai Y. Unique responses of Helicobacter pylori to exogenous hydrophobic compounds. Chem Phys Lipids. 2020. https://doi.org/10.1016/j.chemphyslip.2020.104908
Shimomura H, Hosoda K, Hayashi S, Yokota K, Oguma K, Hirai Y. Steroids mediate resistance to the bactericidal effect of phosphatidylcholines against Helicobacter pylori. FEMS Microbiol Lett. 2009;301:84–94.
Hosoda K, Shimomura H, Hayashi S, Yokota K, Hirai Y. Steroid hormones as bactericidal agents to Helicobacter pylori. FEMS Microbiol Lett. 2011;318:68–75.
McGee DJ, George AE, Trainor EA, Horton KE, Hildebrandt E, Testerman TL. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37. Agents Chemother. 2011;55:2897–904.
Trainor EA, Horton KE, Savage PE, Testerman TL, McGee DJ. Role of the HefC efflux pump in Helicobacter pylori cholesterol-dependent resistance to ceragenins and bile salts. Infect Immun. 2011;79:88–97.
Correia M, Casal S, Vinagre J, Seruca R, Figueiredo C, Touati E, Machado JC. Helicobacter pylori’s cholesterol uptake impacts resistance to docosahexaenoic acid. Int J Med Microbiol. 2014;304:314–20.
Amgalanbaatar A, Shimomura H, Hosoda K, Hayashi S, Yokota K, Hirai Y. Antibacterial activity of a novel synthetic progesterone species carrying a linoleic acid molecule against Helicobacter pylori and the hormonal effect of its steroid on a murine macrophage-like cell line. J Steroid Biochem Mol Biol. 2014;140:17–25.
Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177:5327–33.
Lebrun AH, Wunder C, Hildebrand J, Churin Y, Zähringer U, Lindner B, Meyer TF, Heinz E, Warnecke D. Cloning of a cholesterol-α-glucosyltransferase from Helicobacter pylori. J Biol Chem. 2006;281:27765–72.
Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zähringer U, Mollenkopf HJ, Heinz E, Meyer TF. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–8.
Lee H, Wang P, Hoshino H, Ito Y, Kobayashi M, Nakayama J, Seeberger PH, Fukuda M. α1,4-GlcNAc-capped mucin-type O-glycan inhibits cholesterol α-glucosyltransferase from Helicobacter pylori and suppress. H pylori growth Glycobiol. 2008;18:549–58.
Hoshino H, Tsuchida A, Kametani K, Mori M, Nishizawa T, Suzuki T, Nakamura H, Lee H, Ito Y, Kobayashi M, Masumoto J, Fujita M, Fukuda M, Nakayama J. Membrane-associated activation of cholesterol α-glucosyltransferase, an enzyme responsible for biosynthesis of cholesteryl-α-D-glucopyranoside in Helicobacter pylori critical for its survival. J Histochem Cytochem. 2011;59:98–105.
Jan HM, Chen YC, Shih YY, Huang YC, Tu Z, Ingle AB, Liu SW, Wu MS, G-Hague J, Mong KKT, Chen YR, Lin CH. Metabolic labeling of cholesteryl glucosides in Helicobacter pylori reveals how the uptake of human lipids enhances bacterial virulence. Chem Sci 2016;7:6208–16.
Beining PR, Huff E, Prescott B, Theodore TS. Characterization of the lipids of mesosomal vesicles and plasma membranes from Staphylococcus aureus. J Bacteriol 1975;121:137–43.
Yu X, Liu T, Zhu F, Khosla C. In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli. PNAS 2011;108:18643–8.
Huang W, Jia J, Edwards P, Dehesh K, Schneider G, Lindqvist Y. Crystal structure of b-ketoacyl-acyl carrier protein synthase II from E. coli reveals the molecular architecture of condensing enzymes. EMBO J. 1998;17:1183–91.
Kelly DJ, Hughes NJ. In: Mobley HLT, Mendz GL., Hazell SL. (Ed.), Chapter 12 the citric acid cycle and fatty acid biosynthesis. Helicobacter pylori: Physiology and Genetics. ASM Press, Washington (DC); 2001.
Funding
This work was supported by a Grant-in-Aid from Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP), JSPS KAKENHI (Grant Number 23K04952), and JKA promotion funds from KEIRIN RACE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wanibuchi, K., Hosoda, K., Amgalanbaatar, A. et al. Aspects for development of novel antibacterial medicines using a vitamin D3 decomposition product in Helicobacter pylori infection. J Antibiot 76, 665–672 (2023). https://doi.org/10.1038/s41429-023-00651-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00651-w