Abstract
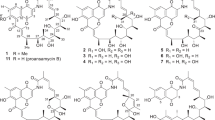
The pluramycin family of antibiotics comprises angucycline compounds derived from actinomycetes that possess anticancer and antibacterial properties. Pluramycins are structurally characterized by two aminoglycosides linked by a carbon-carbon bond next to the γ-pyrone angucycline backbone. Kidamycins (3, 4) and rubiflavins (6–9) were screened through liquid chromatography–mass spectrometry analysis of the crude extracts of Streptomyces sp. W2061, which was cultured in complex media under phosphate-limiting conditions. Newly isolated rubiflavin G (7) and photoactivated compounds (8, 9) were characterized using exhaustive 1D and 2D nuclear magnetic resonance analysis. The cytotoxicity of kidamycin (3), photokidamycin (4), and photorubiflavin G (8) was determined using two human breast cancer cell lines–MCF7 and MDA-MB-231. Compared to MCF7 cells, MDA-MB-231 cells were more sensitive to the active compounds, and photokidamycin (4) considerably inhibited MCF7 and MDA-MB-231 cell growth (IC50 = 3.51 and 0.66 μM, respectively).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Martin JF, Demain AL. Control of antibiotic biosynthesis. Microbiol Rev. 1980;44:230–51.
Demain AL, Sanchez S. Microbial drug discovery: 80 years of progress. J Antibiot. 2009;62:5–16.
van Wezel GP, McDowall KJ. The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Nat Prod Rep. 2011;28:1311–33.
Qin Y, et al. OSMAC strategy integrated with molecular networking discovery peniciacetals A-I, nine new meroterpenoids from the mangrove-derived fungus Penicillium sp. HLLG-122. Bioorg Chem. 2023;130:106271.
Hemphill CFP, et al. OSMAC approach leads to new fusarielin metabolites from Fusarium tricinctum. J Antibiot. 2017;70:726–32.
Maeda K, et al. A new antitumor substance, pluramycin; studies on antitumor substances produced by Actinomycetes. XI. J Antibiot. 1956;9:75–81.
Schmitz H, Crook KE Jr., Bush JA. Hedamycin, a new antitumor antibiotic. I. Production, isolation, and characterization. Antimicrob Agents Chemother. 1966;6:606–12.
Kanda N. A new antitumor antibiotic, kidamycin. I. Isolation, purification and properties of kidamycin. J Antibiot. 1971; 24:599–606.
Jackson M, et al. Altromycins, novel pluramycin-like antibiotics. I. Taxonomy of the producing organism, fermentation and antibacterial activity. J Antibiot. 1990;43:223–8.
Aszalos A, Jelinek M, Berk B. Rubiflavin, a toxic antitumor antibiotic. Antimicrob Agents Chemother. 1964;10:68–74.
Bililign T, Griffith BR, Thorson JS. Structure, activity, synthesis and biosynthesis of aryl-C-glycosides. Nat Prod Rep. 2005;22:742–60.
Cairns MJ, Murray V. Detection of protein-DNA interactions at beta-globin gene cluster in intact human cells utilizing hedamycin as DNA-damaging agent. DNA Cell Biol. 1998;17:325–33.
Masuma R, Tanaka Y, Tanaka H, Omura S. Production of nanaomycin and other antibiotics by phosphate-depressed fermentation using phosphate-trapping agents. J Antibiot. 1986;39:1557–64.
Tanaka Y, Fermentation processes in screening for new bioactive substances. In: Omura S, editors. The Search for Bioactive Compounds from Microorganisms. New York: Springer-Verlag; 1992. p. 303–63.
Heo KT, Lee B, Jang JH, Hong YS. Elucidation of the di-C-glycosylation steps during biosynthesis of the antitumor antibiotic, kidamycin. Front Bioeng Biotechnol. 2022;10:985696.
Martin JF, Rodriguez-Garcia A, Liras P. The master regulator PhoP coordinates phosphate and nitrogen metabolism, respiration, cell differentiation and antibiotic biosynthesis: comparison in Streptomyces coelicolor and Streptomyces avermitilis. J Antibiot. 2017;70:534–41.
Omura S, Tomoda H, Xu QM, Takahashi Y, Iwai Y. Triacsins, new inhibitors of acyl-CoA synthetase produced by Streptomyces sp. J Antibiot. 1986;39:1211–18.
Andreas Fredenhagen US. The Structures of some products from the photodegradation of the pluramycin antibiotics hedamycin and kidamycin. Helv. Chim. Acta. 1985; 68:391–402.
Tsukahara K, Toume K, Ito H, Ishikawa N, Ishibashi M. Isolation of beta-indomycinone guided by cytotoxicity tests from Streptomyces sp. IFM11607 and revision of its double bond geometry. Nat Prod Commun. 2014;9:1327–28.
Mabit T, et al. Total Synthesis of gamma-indomycinone and kidamycinone by means of two regioselective Diels-Alder reactions. J Org Chem. 2017;82:5710–19.
Ando Y, Maezawa Y, Shimura J, Kitamura K, Matsumoto T, Suzuki K. Toward pluramycins with epoxy side chain: Syntheses of kidamycinone and epoxykidamycinone (saptomycinone H). Chem Asian J. 2020;15:828–32.
Hertweck C, Luzhetskyy A, Rebets Y, Bechthold A. Type II polyketide synthases: gaining a deeper insight into enzymatic teamwork. Nat Prod Rep. 2007;24:162–90.
Williamson RT, McDonald LA, Barbieri LR, Carter GT. In support of the original medermycin/lactoquinomycin A structure. Org Lett. 2002;4:4659–62.
Jiang YJ, et al. Medermycin-type naphthoquinones from the marine-derived Streptomyces sp. XMA39. J Nat Prod. 2018;81:2120–24.
Tatsuta K, Ozeki H, Yamaguchi M, Tanaka M, Okui T, Nakata M. Total synthesis and biological evaluation of unnatural (-)-medermycin [(-)-lactoquinomycin]. J Antibiot. 1991;44:901–2.
Cai X, et al. Identification of a C-glycosyltransferase Involved in medermycin biosynthesis. ACS Chem Biol. 2021;16:1059–69.
Zhou B, Jiang YJ, Ji YY, Zhang HJ, Shen L. Lactoquinomycin C and D, two new medermycin derivatives from the marine-derived Streptomyces sp. SS17A. Nat Prod Res. 2020;34:1213–18.
Narita M, Asaoka T, Yano K, Kukita K, Yokoi K, Nakajima T. Novel antibiotic SS21020D and production thereof. Japan Patent Office JPS61115081A. 1986. https://patents.google.com/patent/JPS61115081A/en.
Nadig H, Séquin U, Bunge RH, Hurley TR, Murphey DB, French JC. Isolation and structure of a new antibiotic related to Rubiflavin A. Helv Chim Acta. 1985;68:953–957.
Abe N, Enoki N, Nakakita Y, Uchida H, Nakamura T, Munekata M. Deacetylation effect of N,N-dimethylvancosamine in saptomycins D and E. J Antibiot. 1993;46:692–7.
Abe N, Enoki N, Nakakita Y, Uchida H, Nakamura T, Munekata M. Novel antitumor antibiotics, saptomycins. II. Isolation, physico-chemical properties and structure elucidation. J Antibiot. 1993;46:1536–49.
Fredenhagen A, Sequin U. The photodeactivation of hedamycin, an antitumor antibiotic of the pluramycin type. J Antibiot. 1985;38:236–41.
Kitamura K, Ando Y, Matsumoto T, Suzuki K. Total synthesis of aryl C-glycoside natural products: Strategies and tactics. Chem Rev. 2018;118:1495–1598.
Bililign T, Hyun CG, Williams JS, Czisny AM, Thorson JS. The hedamycin locus implicates a novel aromatic PKS priming mechanism. Chem Biol. 2004;11:959–69.
Acknowledgements
This work was supported by the Basic Science Research Program (NRF 2020R1I1A206871314) of the Ministry of Education, the NRF grant and the KRIBB Research Initiative Program (KGM5292322 and KGM1222312) funded by the Ministry of Science and ICT (MSIT) of the Republic of Korea. We thank the Korea Basic Science Institute (KBSI), Ochang, Korea, for providing the NMR (Bruker).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, B., Lee, GE., Hwang, G.J. et al. Rubiflavin G, photorubiflavin G, and photorubiflavin E: Novel pluramycin derivatives from Streptomyces sp. W2061 and their anticancer activity against breast cancer cells. J Antibiot 76, 585–591 (2023). https://doi.org/10.1038/s41429-023-00643-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00643-w