Abstract
In a healthy gut microbiota, short chain fatty acids (SCFAs) are produced. The antibacterial action of SCFAs against intestinal pathogens makes them useful for ensuring the safety of food and human health. In this study, we aimed to assess the in vitro inhibitory activity of SCFAs, and to report, for the first time, their impact on the activity of new β-lactam/β-lactamase inhibitor combinations. The minimum inhibitory concentrations of acetic, propionic, and butyric acids were determined against E. coli clinical isolates recovered from gastrointestinal infections. Cefoperazone/sulbactam, ceftazidime/avibactam and cefepime/enmetazobactam are new β-lactam/β-lactamase inhibitor combinations that were studied for their combined therapeutic effects. Also, the effects of pH and concentration of SCFAs were evaluated on in vitro bacterial growth and expression of genes encoding for motility, adhesion, invasion, and biofilm formation. SCFAs were tested at concentrations of 12 mM at pH 7.4 (ileum-conditions), in addition to 60 mM and 123 mM, at pH 6.5 (colon-conditions). The tested SCFAs showed the same MIC (3750 μg ml−1 ≃ 60 mM) against all isolates. Furthermore, the addition of SCFAs to the tested β-lactam/β-lactamase inhibitor combinations greatly restored the susceptibility of the isolates. SCFAs had significant effect on bacterial growth and virulence in a pH and concentration-dependent manner; low ileal concentration potentiated E. coli growth, while higher colonic concentration significantly suppressed growth and down-regulated the expression of virulence genes (fliC, ipaH, FimH, BssS). Therefore, the significant inhibitory effect of colonic SCFAs on β-lactam/β-lactamase inhibitor combinations might lead to the development of promising treatment strategies.
Key points
-
β-lactamase inhibitors restore the susceptibility of E. coli isolates.
-
SCFAs had inhibitory effect on E. coli growth and virulence from GIT infections.
-
Effect of SCFAs on the activity of β-lactam/β-lactamase combinations of interest.
Similar content being viewed by others
Introduction
Escherichia coli is an important member of family Enterobacteriaceae that is responsible for many intestinal and extra-intestinal infections in human [1]. E. coli strains have an arsenal of virulence factors, which allows them to cause a broad spectrum of infections including urinary tract infections, gastroenteritis, pneumonia, septicemia, and meningitis [2]. Virulence factors are produced by pathogenic bacteria and are encoded by specific genes located on chromosomes or mobile genetic elements [3]. Bacterial cell surface proteins and secreted factors make up the two categories of E. coli’s virulence factors. Cell surface virulence factors most commonly include fimbriae that help in adhesion to host cell surface, tissue invasion, biofilm formation and cytokine induction. In addition, cell surface virulence factors include flagella which are responsible of motility. A couple of examples of secreted virulence factors are hemolysin and siderophores [4, 5].
E. coli infections can be associated with high mortality rates if not treated properly [6]. Beta-lactam antibiotics such as cephalosporins are the most widely used antimicrobials for treating E. coli infections [7]. However, the extensive use of antibiotics has promoted the rapid development of multidrug resistant (MDR) isolates, which have become a global health problem especially in developing countries [8]. β-lactamase enzymes are the major contributors for cephalosporins resistance, for example, extended spectrum β-lactamases (ESBLs) have activity against 3rd and 4th generation cephalosporins in addition to monobactams [9, 10]. Recently, new combinations between cephalosporins and β-lactamase inhibitors have been found to exhibit synergistic activities against MDR organisms, these combinations include cefoperazone/sulbactam, ceftazidime/avibactam and cefepime/enmetazobactam [11, 12]. Ceftazidime/avibactam was approved in the USA in 2015 and in Europe in 2016, cefoperazone/sulbactam is currently approved in some European countries, but not in the USA, while cefepime/enmetazobactam is still to be approved [13]. Although, these new β-lactamase inhibitors were able to enhance the activity of cephalosporins against many clinical MDR E. coli isolates in vitro, some of the tested strains remained resistant to such combinations [14]. This points to the need to search for other solutions to such resistance.
Organic acids have a long history of use in the food industry as food preservatives, in particular short chain fatty acids (SCFAs), such as acetic acid (AA), butyric acid (BA), and propionic acid (PA), which are naturally produced by microbiota of the ileum (i-SCFA) and the colon (c-SCFA) [15]. The amount and type of SCFAs produced are influenced by the diet intake, in addition to the composition of the gut microbiota [16]. The amount of total SCFAs in the large intestine ranges from 60 to 123 mM, with acetate, propionate, and butyrate having a molar ratio of 60:20:20, while in the human ileum, lower concentrations of SCFAs (from 7 to 20 mM) have been observed [17]. SCFAs exert several beneficial effects on host metabolism and immune system [18]. They have also been shown to modulate replication, colonization, and virulence of enteric pathogens [19]. For these reasons, SCFAs are of increasing interest for development of new therapeutics.
The aim of current study is to clarify the role of short chain fatty acids (SCFAs) on growth and virulence of Escherichia coli clinical isolates recovered from gastrointestinal infections, and to investigate the therapeutic potentials of short chain fatty acids together with the three tested β-lactam/β-lactamase combinations.
Materials and methods
Bacterial isolates
In this study, 140 Escherichia coli isolates were previously obtained from the laboratory of the Gastrointestinal Surgery Center (GISC) in Mansoura, Egypt. These isolates were identified by standard microbiological methods. The isolates were stored at −20 °C in tryptone soya broth (TSB, Oxoid, UK) with 20% glycerol [20].
Susceptibility of E. coli isolates to β-Lactam/β-Lactamase inhibitor combinations
Three different β-lactam/β-lactamase inhibitor combinations were examined for their antimicrobial activity including cefoperazone/sulbactam, ceftazidime/avibactam, and cefepime/enmetazobactam. Cefoperazone/sulbactam and ceftazidime/avibactam were used at ratios of 1:2, and 4:1, respectively. A fixed concentration of enmetazobactam (8 μg ml−1) was used in cefepime/enmetazobactam combination [14]. The Clinical and Laboratory Standards Institute’s broth microdilution method was used to determine minimum inhibitory concentrations (MICs) [21].
Sigma Aldrich (Darmstadt, Germany) provided the test antibiotics: cefoperazone, ceftazidime, and cefepime. MedChemExpress MCE (Monmouth Junction, USA) provided the three β-lactamase inhibitors. All powders were kept at 4 °C in airtight containers. The lowest level of β-lactam antibiotic at which no visible bacterial growth detected was considered as the MIC. Resazurin dye (Sigma-Aldrich, Darmstadt, Germany) was used to detect bacterial growth colorimetrically producing pink, fluorescent resofurin as a byproduct [22].
Susceptibility of E. coli isolates to different SCFAs singly and in combinations
The MICs of acetic acid (Sigma Aldrich, Schnelldorf, Germany), propionic acid and butyric acid (Alfa Aesar, ThermoFisher Scientific, Massachusetts, Waltham, MA, USA) were determined by broth microdilution method according to Clinical and Laboratory Standards Institute [21].
Susceptibility of E. coli isolates for combined therapy of new β-lactam/β-lactamase inhibitor along with SCFAs
The MICs for β-lactam/β-lactamase inhibitor combinations were determined in presence of sub-MIC (½ MIC) of SCFAs at pH 6.5 using the broth microdilution method [21]. Starting with a double strength β-lactam/β-lactamase inhibitor concentration, two-fold serial dilutions were made in microtiter wells leaving one well as growth control and another as negative control. Then ½ the MIC of SCFAs was added (37.5 μl from 10 mg μl−1 stock) to each well except for the growth and the negative control (un-inoculated) wells. Finally, 100 μl of bacterial suspension (1.5 × 105 CFU ml−1) were added and plates were incubated for 24 h at 37 °C and the MIC was recorded after adding resazurin dye.
The effect of SCFAs on in vitro bacterial growth of E. coli
A 24-h well isolated E. coli colony on nutrient agar was used to inoculate 10 ml of MHB that was incubated for overnight at 37 °C. Then, one ml of the incubated culture was used to inoculate 50 ml of sterile MHB in a 100-ml conical flask. The flask was incubated at 37 °C in shaking incubator. One-ml aliquots of the culture were taken for measuring the optical density (OD) at wavelength of 600 nm and for viable counting after 2 h and every 60 min. The viable count defined as the number of colony-forming units (CFU) was determined using the standard plate counting technique [23].
To investigate the effect of SCFAs on bacterial growth, a mixture of acetic, propionic, and butyric acid (with a ratio of 60:20:20 for acetate, propionate, and butyrate; respectively) was added to bacterial suspension of E. coli isolates -that remained resistant to β-lactams even after the addition of β-lactamase inhibitors- so as to mimic both ileum and colonic conditions [16]. Similarly, OD was measured after 2 h and every 60 min. At the end, growth curves were drawn for the bacterial isolate in the presence and absence of SCFAs.
Effect of SCFAs on transcription of virulence genes by quantitative real–time polymerase chain reaction (qRT-PCR)
Fifteen-milliliter falcon tubes were filled with 10 ml MHB and 100 μl of bacterial suspension (104 CFU ml−1) from an overnight culture of the test isolate. Then various concentrations of SCFAs were made in the inoculated falcon tubes as follows; i-SCFA (12 mM at pH 7.4) and c-SCFA (60 mM and 123 mM at pH 6.5) or sterile NaCl (Control). The prepared cultures were incubated to mid log phase at 37 °C in orbital shaker then centrifuged at 2000 × g for 10 min. The supernatant was discarded, and the pellet was resuspended in 250 μl of TE buffer (10 mM Tris pH 8 and 1 mM EDTA), then 25 μl of 10% Sodium dodecyl sulfate (SDS) was added. The mixture was incubated at 65 °C for 30 min, then 1 ml of trizol was added and the sample was sonicated on ice-bath using probe sonicator (Thermofisher Scientific, CA, USA,) for 2 min [24].
Total RNA was extracted using Qiagen RNA Protect-RNeasy Kit (Qiagen, Hilden, Germany) according to manufacturer’s recommendations. Immediately, RNA concentration and purity was evaluated using Thermo Scientific™ NanoDrop™ One Microvolume UV-Vis Spectrophotometer (Thermofisher Scientific, CA, USA,). RNA was reverse transcribed to cDNA using RevertAid First Strand cDNA Synthesis kit according to manufacture instructions (Biogen, Munich, Germany).
Table 1 demonstrated the primers for the four tested virulence associated genes fimH, ipaH, fliC, and BssS that were analyzed using SensiFAST™ SYBR® High-ROX Kit (Bioline USA Inc., USA). The 10 μl total volume of qRT-PCR reaction was made up of 1 μl of cDNA, 0.7 μl of each forward and reverse primers (10 μM), 5 μl of 2× SYBR Green Master Mix, 1 μl of QN ROX Reference Dye and 1.6 μl of nuclease-free water. The amplification conditions were made as follows: initial denaturation at 95 °C for 20 s, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s, extension at 72 °C for 30 s, and a final melting curve program of 15 s at 95 °C, 60 s at 60 °C, followed by a dissociation step for 15 s at 95 °C. The reaction was carried out in StepOne™ Real-Time PCR System (Applied Biosystems™, ThermoFisher Scientific, Foster, CA, USA). The comparative quantification method (∆Ct) was used to determine the up-and down-regulated genes, where the expression level of targeted genes in comparison to the control 16 S rRNA gene (Sigma Co., USA) was calculated by using the equation RQ = 2-∆∆CT where ∆∆Ct = (Cttarget genes – Ct16srRNA)treatment − (Cttarget genes − Ct16srRNA)control, where Ct is the threshold cycle.
The effect of SCFAs on E. coli motility
The effect of SCFAs on the swarming motility of E. coli isolates was carried out in semisolid agar plates. E. coli isolates were grown in Luria-Bertani (LB) broth overnight at 37 °C. Soft agar plates (1% tryptone, 0.5% NaCl, 0.25% agar) were prepared the day before assay. The control plates had the same quantity of saline as the total SCFAs, and SCFAs were added to the agar medium immediately before pouring into the plate. Three μl of E. coli overnight cultures were added to the center of each plate. Finally, plates were incubated at 37 °C for 10 h. The experiment was carried out in triplicate [16].
The Effect of SCFAs on biofilm formation of E. coli isolates
The biofilm formation was performed as described by Salo et al. [25]. Briefly, 5 ml of LB broth was inoculated with a test isolate colony and incubated at 37 °C for 18–20 h. Bacterial cells were collected by centrifugation at 5000 rpm for 5 min, the cells were washed with 0.5 ml phosphate buffered saline, followed by centrifugation. The washed cells were resuspended in 1 ml of LB broth and the cell density was adjusted to 107 cells ml−1. 200 μl of each test isolate were placed into the round bottomed wells of 96-well polystyrene microtiter plate. SCFAs were added to the test isolates at concentrations of 60 mM and 123 mM and plates were incubated at 37 °C for 24 h. The bacterial suspensions were gently aspirated from the wells that were washed three times each with 200 μl of sterile PBS to remove planktonic cells. The plates were then dried for 45 min at room temperature and 150 μl of 0.4% crystal violet (CV) was added to each well and left for 45 min at room temperature. After this, 150 μl of 95% ethanol was added to each well and left for 45 min. Then, 100 μl of each well was transferred to a fresh 96-well microtiter plate to measure the absorbance at 600 nm using a Synergy HT microtiter plate reader (BioTek Instruments, Winooski, VT, USA),.
The average OD of three wells for each strain was computed (ODt), and the cut-off OD (ODc) was determined as being three standard deviations above the average OD of the negative control. The test isolates were classified as non-biofilm forming (N): ODt ≤ ODc, weak biofilm forming (W): ODc < ODt ≤ 2 × ODc, moderate biofilm forming (M): 2 × ODc < ODt ≤ 4 × ODc and strong biofilm forming isolates (S): ODt > 4 × ODc [26].
The effect of SCFAs on E. coli adhesion
Bacterial adhesion was evaluated by the same technique performed with biofilm production, but instead of incubating the 96-well plates for 24 h, plates were incubated for just 2 h and the procedure was continued as before [27].
Statistical analysis
GraphPad Prism software was utilized for the statistical analysis employing the one-way ANOVA test (version 7.0, GraphPad Software Inc., La Jolla, CA, USA). Statistics are deemed significant for P-values below 0.05. All data were expressed as mean ± SEM.
Results
Susceptibility of E. coli isolates to β-lactam antibiotic in presence of β-lactamase inhibitors
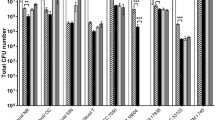
Figure 1 shows that cephalosporins were effective against E. coli isolates at the following rates: cefoperazone (52.9%), ceftazidime (43.5%), and cefepime (32.1%). The addition of β-lactamase inhibitors restored the susceptibility of the majority of E. coli isolates, where 94.3%, 89.2%, 85.7% of the isolates became susceptible to cefoperazone/sulbactam, cefepime/enmetazobactam and ceftazidime/avibactam, respectively. Eighteen E. coli isolates remained resistant or had intermediate resistance to β-lactams even after the addition of β-lactamase inhibitors. These isolates were used in the next experiments.
Susceptibility of resistant E. coli isolates to different short chain fatty acids (SCFAs)
Acetic, propionic, and butyric acids showed the same MIC (3750 μg ml−1) against all tested isolates. Where, 3750 μg ml−1 of SCFAs is approximately equivalent to 60 mM, which is the lowest concentration of SCFAs normally present in human colon.
Investigating the combined effect of β-lactams/β-lactamase inhibitors along with SCFAs
New combinations that included SCFAs along with cephalosporins and new β-lactamase inhibitors were examined for their therapeutic effect against the eighteen E. coli isolates that remained resistant. Table 2 shows the susceptibility of these isolates to each of the three combinations alone and in presence of SCFAs as interpreted according to CLSI guidelines [21].
Table 3 shows that the addition of SCFAs enhanced the susceptibility of the tested isolates (n = 18) reaching 94.4%, 83.3% and 66.7% for ceftazidime/avibactam, cefoperazone/sulbactam and cefepime/enmetazobactam, respectively. Only one isolate (strain no. 3) remained resistant to cefoperazone/sulbactam and ceftazidime/avibactam after the addition of SCFAs.
The addition of SCFAs resulted in a decrease in the MICs of β-lactam/β-lactamase inhibitor combinations. Our results showed that, after the addition of SCFAs to cefoperazone/sulbactam combination, only 3 out of 14 isolates that were resistant to 32 μg ml−1 remained resistant, and the number of susceptible isolates (MIC ≤ 16 μg ml−1) reached 15 isolates. Furthermore, only one isolate remained resistant to ceftazidime/avibactam combination after the of addition of SCFAs, and the number of isolates having a MIC of less than 0.25 μg ml−1 increased from 2 to 10 isolates. When SCFAs were combined with cefepime/enmetazobactam, ten isolates reported MIC values of less than 0.25 μg ml−1 and all of the tested isolates were inhibited by MIC ≤ 16 μg ml−1 (Table S1).
As shown in Fig. 2, and following combination, the MIC values of β-lactams dramatically decreased according to statistical analysis, and SCFAs were significantly more effective against most clinical isolates of E. coli. The addition of the SCFAs to the three tested combinations significantly enhanced their inhibitory activities with P-values of 0.0001 for cefoperazone/sulbactam, 0.0118 for ceftazidime/avibactam, and 0.0295 for cefepime/enmetazobactam.
Effect of SCFAs on in vitro growth of E. coli
Figure S1 shows the growth curve of a control E. coli isolate (without addition of SCFAs) as measured by optical density (OD) at 600 nm. The collinearity between the viable cell number, as CFU, and the optical density was established in a preliminary calibration curve (Fig. S2).
A representative of the eighteen combinations-resistant isolates were tested for their growth capacity in the presence of different amounts of SCFAs at different pH [12 mM at pH of 7.4 (ileum conditions), 60 mM and 123 mM, at pH of 6.5 (colon conditions)]. The data represented in Fig. 3 and Table S2 -compared to control- revealed that SCFAs had a great impact on the bacterial growth in a pH-dependent and concentration-dependent manner. Growth curve showed that E. coli exhibited increased growth at the low ileal concentration (i-SCFA 12 mM at pH 7.4). On the other hand, high colonic concentration (123 mM c-SCFAs at pH 6.5) significantly suppressed E. coli growth, while low colonic concentration (60 m M) had no significant effect on growth.
A Concentration-dependent effect of SCFAs on growth of representative E. coli isolate (no. 1); measured by OD at 600 nm. 1: [12 mM], 2: [Control], 3: [60 mM], 4: [123 mM]. B Statistical analysis of the effect of different concentration of SCFAs on E. coli growth, P < 0.05 considered statistically significant. All data were expressed as mean ± SE
The effect of short chain fatty acids on expression of E. coli virulence genes
The effect of SCFAs on the expression of four virulence genes encoding for; motility (fliC), adhesion (FimH), invasion (ipaH) and biofilm formation (BssS) were evaluated. The results showed that SCFAs were able to reduce virulence genes expression in E. coli (Table 4, Fig. 4).
Virulence genes transcription showed that c-SCFAs treatment downregulated all tested virulence genes in a concentration-dependent manner. It was found that ipaH and fliC genes were down regulated by 0.6-fold after treatment with 60 mM SCFAs, while BssS and FimH were down regulated by only 0.2 and 0.3-fold; respectively. However, the high-level concentration of SCFAs (123 mM) suppressed the expression of all tested genes, where BssS gene expression was decreased by 0.7-fold, followed by fliC and FimH genes that were suppressed by 0.8-fold, while ipaH became the least expressed gene by 0.9-fold.
On the other hand, E. coli isolates showed overexpression of all tested genes in presence of i-SCFAs (12 mM at pH 7.4). Where the expression of FimH and ipaH genes was increased by 0.5 and 0.4-fold; respectively while both BssS and fliC genes were up regulated by 0.3-fold.
SCFAs modulate E. coli motility
The swarming motility of E. coli was evaluated in the presence of c-SCFAs at different concentrations using motility assay test, where agar plates contained different concentrations of SCFAs (Fig. 5). The swarming motility of E. coli was completely inhibited at 60 mM and 123 mM c-SCFAs compared to controls.
The effect of SCFAs on biofilm formation
Biofilm formation of E. coli isolates shown in Table 5 revealed that 61% of tested eighteen isolates were strong biofilm forming and 39% of isolates were moderate. In the presence of SCFAs at 60 mM, all the tested isolates were moderate biofilm producers, while at 123 mM all isolates were weak biofilm producers.
Effect of SCFAs on bacterial adhesion
The result shown in Table 6 revealed that all tested E. coli clinical isolates can strongly adhere to polystyrene microtiter plate. However, in the presence of 60 mM of SCFAs all the isolates appeared as moderately adherent. In the presence of 123 mM of SCFAs, around 72% of the isolates were found to be weakly adherent, and 5 out of 18 became non-adherent.
Discussion
β-lactam antibiotics are considered the appropriate choices for treating E. coli infections. The inappropriate use that prevails in developing nations have a significant impact on rising rates of resistance and, as a result, rising rates of therapeutic failure [5, 26, 28, 29].
One of the main mechanisms of resistance to β-lactams is the production of β-lactamase enzymes, therefore, β-lactam/β-lactamase inhibitor combinations are particularly valuable treatment options. However, bacterial evolution has progressed and MDR bacteria such as carbapenem-resistant Enterobacteriaceae (CRE), are proliferating [30]. In a recent study, it was found that several cephalosporins, notably cefoperazone (52.9%), ceftazidime (43.5%), and cefepime (32.1%), had high rates of resistance when used alone. Furthermore, elevated resistance rates were noticed with older β-lactam/β-lactamase inhibitor combinations such as amoxicillin/clavulanate and ampicillin/sulbactam (40.7% and 42.9%), respectively [14].
Fortunately, the in vitro activity of some β-lactams was potentiated by addition of new β-lactamase inhibitors, for example, the activity of cefoperazone was enhanced by addition of sulbactam. In the present study, it was found that cefoperazone resistance was declined from 52.9% to 2.2%. Similarly, a study by Ku and Yu [31], demonstrated that addition of sulbactam to cefoperazone significantly enhanced the antimicrobial activity against Gram-negative pathogens.
In the present study, high percentage of tested E. coli isolates (43.5%) were found to be resistant to ceftazidime. Though, most of our isolates (94.3%) were inhibited by the ceftazidime/avibactam combination. Recently, Yang et al. [32] reported that 78% of their isolates were susceptible to ceftazidime/avibactam. In addition, avibactam-ceftazidime combination had demonstrated high activity against P. aeruginosa, and CRE isolates [11].
Furthermore, it was found that enmetazobactam has increased the activity of cefepime, from 43.6% to 85.7%. Similarly, Morrissey et al. [33] reported that enmetazobactam caused a significant reduction in cefepime-MIC90, and suggested that this combination might be a valuable option for empirical treatment of serious Gram-negative infections.
SCFAs are of increasing interest for therapeutics development as they have been shown to modulate replication, colonization, and virulence of enteric pathogens [19]. Intestinal SCFAs are by-products of bacterial fermentation. Most microorganisms are susceptible to antimicrobial effects in presence of organic acids [34]. It was proposed that the reason behind their antimicrobial activity, is the ability to diffuse across cell membrane into the bacterial cytoplasm, modifying the intracellular pH and metabolism [35]. It was reported that the concentrations of SCFAs in colon are 10-fold higher than that in ileum, with noticeable differences in luminal pH [36, 37].
Till now, there have been limited studies evaluating the MIC values of SCFAs in food-borne pathogens [38]. In the current study, we investigated the effect of SCFAs with their different concentrations -normally present in colon- on the activity of new β-lactam/β-lactamase inhibitor combinations in addition to their role in growth and virulence of E. coli isolated from GIT infections. In this study, the MICs of the three most predominant SCFAs were determined. Acetic, propionic, and butyric acids were found to have the same MIC value (3750 μg ml−1) in all tested isolates. This is consistent with a previous study by Lamas et al. [19].
Similarly, another study that evaluated the antimicrobial activity of acetic acid in several pathogens including E. coli, found that the MIC were between 1600 μg ml−1 and 3100 μg ml−1 [39]. Another study showed that organic acids could prevent growth of E. coli and Salmonella isolates at varying concentrations [40]. In addition, MICs of 15 mg ml−1 and 10 mg ml−1 of acetic acid were reported against E. coli and Salmonella; respectively [40].
The present study found that the presence of SCFAs in a concentration that mimic that present normally in the colon, greatly restored the susceptibility of almost E. coli isolates against the tested β-lactams, where only one isolate remained resistant. Moreover, MICs values of β-lactam/β-lactamase inhibitor combinations were significantly decreased after the addition of SCFAs; cefoperazone/sulbactam/SCFAs combination (<0.0001) followed by ceftazidime/avibactam/SCFAs (P-value of 0.0118) then cefepime/enmetazobactam/SCFAs (P-value of 0.0295).
In addition, we examined the effect of SCFAs on the growth and pathogenicity of E. coli isolates recovered from GIT infections in the context of alterations in the microenvironment with regard to concentration and pH. The ileal and colonic conditions were modeled using the concentrations and ratios of SCFAs along with pH values. We demonstrated that the concentration and pH of SCFAs had an impact on the growth of E. coli, where ileal (i-SCFA) favored growth, and colonic (c-SCFA) suppressed growth. These results are in agreement with that of Zhang et al. [16] who found that under ileal conditions (pH = 7.4; 12 mM), SCFAs significantly potentiated the growth and motility of E. coli. However, under colonic conditions (pH = 6.5; 65 to 123 mM), SCFAs significantly inhibited growth in a pH dependent fashion.
We also examined the impact of SCFAs on E. coli virulence genes transcription including those encoding for motility (fliC), adhesion (FimH), invasion (ipaH) and biofilm formation (BssS) [41, 42].
Overexpression of all tested genes was observed in presence of i-SCFAs (12 mM at pH 7.4). On the other hand, c-SCFA down regulated the virulence genes expression in a concentration-dependent manner. These findings are in agreement with Zhang et al. [43], reporting that under ileal conditions, SCFAs significantly potentiated the growth and motility of E. coli. However, in colonic conditions (pH = 6.5; 65–123 mM), SCFAs significantly down-regulated virulence genes expression (fliC, fimH, htrA, chuA, pks), especially those linked to adhesion and motility. The adhesin gene (fimH) and the motility gene (fliC) were both downregulated in 11 out of 15 and 9 out of 15 E. coli strains, respectively.
Moreover, our findings were consistent with those reported with Salmonella and Enterohemorrhagic E. coli (EHEC) isolates. For instance, Lackraj et al. [44] demonstrated that SCFAs simulating the concentration in the small intestine increased the expression of genes involved in EHEC flagella biosynthesis and motility, while doing the opposite for SCFAs simulating the large intestine, where flagella expression by (FliC) confirmed a strong, significant 3.7-fold increase under 30 mM SCFA relative to the 172 mM SCFA treatment, when compared to the respective NaCl controls. Similarly, ileal SCFAs increased but colonic SCFAs reduced the expression of Salmonella virulence genes that code for invasion of epithelial cells and survival within macrophages [26]. Another study found that, Salmonella Typhimurium overexpressed 14 of the genes that were evaluated in the presence of SCFAs as compared to control growth conditions [19].
In summary, significant effort was made in the last ten years to combat the rising resistance rates against classical β-lactams and β-lactam/β-lactamase inhibitor combinations through developing new combinations that target many pathogens including E. coli. Our study confirmed that using new combinations restored the susceptibility of most E. coli isolates. Moreover, the lowest concentration of SCFAs that is normally produced in colon had significant inhibitory effect on bacterial growth and virulence that could help in restoring the susceptibility of isolates to β-lactam antibiotics in a concentration-dependent manner. To the best of our knowledge, this study is the first to report the effect of SCFAs on therapy by β-lactam/β-lactamase inhibitor combinations. In addition, our results could be promising to control enteric pathogens and warrants more in vivo testing to validate their therapeutic effectiveness.
Data availability
Data will be available upon request.
References
Williams KP, Gillespie JJ, Sobral BWS, Nordberg EK, Snyder EE, Shallom JM, et al. Phylogeny of gammaproteobacteria. J Bacteriol [Internet] 2010;192:2305–14. Available from: https://doi.org/10.1128/JB.01480-09. [cited 2022 Jun 8]
Kaper JB, Nataro JP, Mobley HLT. Pathogenic escherichia coli. Nat Rev Microbiol. 2004;2:123–40.
Pakbin B, Brück WM, Rossen JWA. Virulence factors of enteric pathogenic Escherichia coli: A review. Int J Mol Sci. 2021;22:9922.
Shah C, Baral R, Bartaula B, Shrestha LB. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019;19:1–6.
Kadry A, Al-Kashef N, sciences AEGA health, 2020 undefined. Distribution of genes encoding adhesins and biofilm formation capacity among Uropathogenic Escherichia coli isolates in relation to the antimicrobial resistance. ajol info [Internet] 2020;20:238. Available from: https://www.ajol.info/index.php/ahs/article/view/195012 [cited 2022 Jun 8]
Christensen SB. Drugs that changed society: History and current status of the early antibiotics: Salvarsan, sulfonamides, and β-lactams. Molecules 2021;26:6057.
Pitout JDD Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front Microbiol. 2012;3(JAN).
Kadry AA, Serry FM, El-Ganiny AM, El-Baz AM. Integron occurrence is linked to reduced biocide susceptibility in multidrug resistant Pseudomonas aeruginosa. Br J Biomed Sci. 2017;74:78–84.
Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: A clinical update. Clin Microbiol Rev. 2005;18:657–86.
Lee YL, Ko WC, Lee WS, Lu PL, Chen YH, Cheng SH, et al. In-vitro activity of cefiderocol, cefepime/zidebactam, cefepime/enmetazobactam, omadacycline, eravacycline and other comparative agents against carbapenem-nonsusceptible Enterobacterales: Results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) in 2017–2020. Int J Antimicrob Agents. 2021;58:106377.
Papp-Wallace KM. The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections. Expert Opin Pharmacother. 2019;20:2169–84.
Alfei S, Schito AM. β-Lactam Antibiotics and β-Lactamase Enzymes Inhibitors, Part 2: Our Limited Resources. Pharmaceuticals 2022;15:476.
Bassetti M, Garau J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J Antimicrob Chemother. 2021;76:IV23–37.
Kadry AA, El-Antrawy MA, El-Ganiny AM. Management of clinical infections of Escherichia coli by new β-lactam/β-lactamase inhibitor combinations. Iran J Microbiol. 2022;14:466–74.
Bishehsari F, Engen PA, Preite NZ, Tuncil YE, Naqib A, Shaikh M, et al. Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes 2018;9:102.
Zhang S, Dogan B, Guo C, Herlekar D, Stewart K, Scherl EJ, et al. Short chain fatty acids modulate the growth and virulence of pathosymbiont escherichia coli and host response. Antibiotics 2020;9:1–20.
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200.
Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021;66:103293.
Lamas A, Regal P, Vázquez B, Cepeda A, Franco CM. Short chain fatty acids commonly produced by gut microbiota influence Salmonella enterica motility, biofilm formation, and gene expression. Antibiotics 2019;8:265.
Washington C, Stephen A, Janda W Koneman’s color atlas and textbook of diagnostic microbiology. USA: Lippincott williams & wilkins; 2006.
Clinical and Laboratory Standards Institute (2017). Performance standards for antimicrobial disk susceptibility tests; approved standard— Twelfth Edition. CLSI document M02-A11. CLSI, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania, USA.
O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–6.
Brown A, Smith H Benson’s Microbiological Applications, Laboratory Manual in General Microbiology, Short Version. McGraw-Hill Education; 2014.
Villa-Rodríguez E, Ibarra-Gámez C, de Los Santos-Villalobos S. Extraction of high-quality RNA from Bacillus subtilis with a lysozyme pre-treatment followed by the Trizol method. J Microbiol Methods. 2018;147:14–6.
Salo J, Sevander JJ, Tapiainen T, Ikäheimo I, Pokka T, Koskela M, et al. Biofilm formation by Escherichia coli isolated from patients with urinary tract infections. Clin Nephrol. 2009;71:501–7.
Khairy RMM, Fathy ZA, Mahrous DM, Mohamed ES, Abdelrahim SS. Prevalence, phylogeny, and antimicrobial resistance of Escherichia coli pathotypes isolated from children less than 5 years old with community acquired-diarrhea in Upper Egypt. BMC Infect Dis. 2020;20:1–9.
Fletcher M, Pringle JH. The effect of surface free energy and medium surface tension on bacterial attachment to solid surfaces. J Colloid Interface Sci. 1985;104:5–14.
Michael CA, Dominey-Howes D, Labbate M The antimicrobial resistance crisis: Causes, consequences, and management. Front Public Health. 2014 Sep;2(SEP).
Khalifa SM, Abd El-Aziz AM, Hassan R, Abdelmegeed ES. β-lactam resistance associated with β-lactamase production and porin alteration in clinical isolates of E. coli and K. pneumoniae. PLoS One 2021;16:e0251594.5
Toussaint KA, Gallagher JC. Combinations: From Then to Now. Ann Pharm. 2014;49:86–98.
Ku YH, Yu WL. Cefoperazone/sulbactam: New composites against multiresistant gram negative bacteria? Infect Genet Evol. 2021;88:104707.
Yang X, Wang D, Zhou Q, Nie F, Du H, Pang X, et al. Antimicrobial susceptibility testing of Enterobacteriaceae: Determination of disk content and Kirby-Bauer breakpoint for ceftazidime/avibactam. BMC Microbiol. 2019 Nov;19.
Morrissey I, Magnet S, Hawser S, Shapiro S, Knechtle P In Vitro Activity of Cefepime-Enmetazobactam against Gram-Negative Isolates Collected from US and European Hospitals during 2014–2015. Am Soc Microbiol [Internet]. 2019 [cited 2022 Jun 8];63. Available from: https://doi.org/10.1128/AAC.00514-19
Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int J Mol Sci. 2022;23:1105.
Mirzaei R, Dehkhodaie E, Bouzari B, Rahimi M, Gholestani A, Hosseini-Fard SR, et al. Dual role of microbiota-derived short-chain fatty acids on host and pathogen. Biomed Pharmacother. 2022;145:112352.
Cummings JH, Pomare EW, Branch WJ, Naylor CP, MacFarlane G. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221–7.
Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57–64.
Peng M, Biswas D. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Crit Rev Food Sci Nutr. 2017;57:3987–4002.
Sol C, Oddo JM, Puyalto M, Carvajal A, Gómez M, Costillas S, et al. In vitro evaluation of the antimicrobial activity of several short-and medium-chain fatty acid salts and their combinations. J Anim Sci. 2017;95:15.
Halstead FD, Rauf M, Moiemen NS, Bamford A, Wearn CM, Fraise AP, et al. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PloS One. 2015;10:e0136190.
He Y, Xu T, Fossheim LE, Zhang XH FliC, a flagellin protein, is essential for the growth and virulence of fish pathogen Edwardsiella tarda. 2012;
Elhenawy W, Tsai CN, Coombes BK. Host-specific adaptive diversification of Crohn’s disease-associated adherent-invasive Escherichia coli. Cell Host Microbe. 2019;25:301–12.
Zhang S, Dogan B, Guo C, Herlekar D, Stewart K, Scherl EJ, et al. Short chain fatty acids modulate the growth and virulence of pathosymbiont Escherichia coli and host response. Antibiotics 2020;9:462.
Lackraj T, Kim JI, Tran SLY, Foster DEB. Differential modulation of flagella expression in enterohaemorrhagic Escherichia coli O157: H7 by intestinal short-chain fatty acid mixes. Microbiology 2016;162:1761–72.
Osonga FJ, Akgul A, Yazgan I, Akgul A, Ontman R, Kariuki VM, et al. Flavonoid-derived anisotropic silver nanoparticles inhibit growth and change the expression of virulence genes in Escherichia coli SM10. RSC Adv. 2018;8:4649–61.
Kim HR, Eom YB. Synergistic activity of equol and meropenem against carbapenem-resistant Escherichia coli. Antibiotics 2021;10:161.
Persson S, Olsen KEP, Scheutz F, Krogfelt KA, Gerner-Smidt P. A method for fast and simple detection of major diarrhoeagenic Escherichia coli in the routine diagnostic laboratory. Clin Microbiol Infect. 2007;13:516–24.
Acknowledgements
The authors appreciate the assistance of the staff of the Gastrointestinal Surgery Center (GISC), Mansoura University in collecting the clinical specimens. The authors would like to acknowledge Prof. Dr. Fathy Serry (Professor of Microbiology and Immunology, Zagazig University) for proofreading the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AAK contributed to the study conception, data analysis, supervision, review, and editing. MAE-A contributed to methodology, resources, data collection and analysis, writing draft, and editing manuscript. AME-G contributed to the study design, data analysis and validation, writing draft, review and editing of manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental tests were carried according to the approval number (FPDU13/2022) from Faculty of Pharmacy, Delta University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadry, A.A., El-Antrawy, M.A. & El-Ganiny, A.M. Impact of short chain fatty acids (SCFAs) on antimicrobial activity of new β-lactam/β-lactamase inhibitor combinations and on virulence of Escherichia coli isolates. J Antibiot 76, 225–235 (2023). https://doi.org/10.1038/s41429-023-00595-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00595-1
This article is cited by
-
Investigation of plasmid-mediated quinolone resistance among extended-spectrum β-lactamase isolates of E. coli and K. pneumoniae
The Journal of Antibiotics (2024)