Abstract
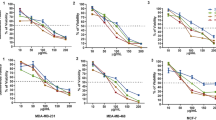
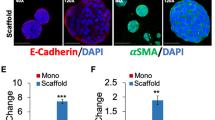
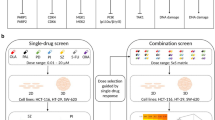
Targeting and eradicating cancer stem cells (CSCs), also termed tumor-initiating cells, are promising strategies for preventing cancer progression and recurrence. To identify candidate compounds targeting CSCs, we established a new screening strategy with colorectal CSC spheres and non-CSC spheres in three-dimensional (3D) culture system. Through chemical screening using our system with in-house microbial metabolite library, we identified polyether cation ionophores that selectively inhibited CSC sphere formation, whereas CSC spheres were resistant to conventional anticancer agents. One of the hit compounds, the most selective and effective microbial metabolite lenoremycin, decreased CSC populations via inducing reactive oxygen species production. This study demonstrated that our newly established screening system is useful for discovering agents that selectively eliminate CSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84.
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–34.
Saygin C, Matei D, Majeti R, Reizes O, Lathia JD. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. 2019;24:25–40.
Takebe N, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–64.
Yang L, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:1–35.
Jang MK, Mashima T, Seimiya H. Tankyrase inhibitors target colorectal cancer stem cells via AXIN-dependent downregulation of c-KIT tyrosine kinase. Mol Cancer Ther. 2020;19:765–76.
Sun HR, et al. Therapeutic strategies targeting cancer stem cells and their microenvironment. Front Oncol. 2019;9:1–14.
Madoux F, et al. A 1536-well 3D viability assay to assess the cytotoxic effect of drugs on spheroids. SLAS Discov. 2017;22:516–24.
Lal-Nag M, et al. A high-throughput screening model of the tumor microenvironment for ovarian cancer cell growth. SLAS Discov. 2017;22:494–506.
Yan X, et al. High throughput scaffold-based 3D micro-tumor array for efficient drug screening and chemosensitivity testing. Biomaterials. 2019;198:167–79.
Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59.
Kakeya H. Natural products-prompted chemical biology: phenotypic screening and a new platform for target identification. Nat Prod Rep. 2016;33:648–54.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Munro MJ, Wickremesekera SK, Peng L, Tan ST, Itinteang T. Cancer stem cells in colorectal cancer: a review. J Clin Pathol. 2018;71:110–6.
Takeda K, et al. Sox2 is associated with cancer stem-like properties in colorectal cancer. Sci Rep. 2018;8:17639.
Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234:8381–95.
Deka J, et al. Bcl9/Bcl9l are critical for Wnt-mediated regulation of stem cell traits in colon epithelium and adenocarcinomas. Cancer Res. 2010;70:6619–28.
Lv J, et al. Cell softness regulates tumorigenicity and stemness of cancer cells. EMBO J. 2020;40:e106123.
Kubota T, Hinoh G, Mayama M, Motokawa K, Yasuda Y. Antibiotic A-130, isolation and characterization. J Antibiot. 1975;28:931–4.
Westley JW. Polyether antibiotics: versatile carboxylic acid ionophores produced by Streptomyces. Adv Appl Microbiol. 1977;22:177–223.
Corbaz VR, et al. Nonactin. Helv Chim Acta. 1955;174:1445–8.
Marrone TJ, Merz KM. Molecular recognition of potassium ion by the naturally occurring antibiotic ionophore nonactin. J Am Chem Soc. 1992;114:7542–9.
Harned RL, Hidy PH, Corum CJ, Jones KL. Nigericin a new crystalline antibiotic from an unidentified Streptomyces. Antibiot Chemother. 1951;1:594–6.
Steinrauf LK, Pinkerton M, Chamberlin JW. The structure of nigericin. Biochem Biophys Res Commun. 1968;33:29–31.
Deng CC, et al. Nigericin selectively targets cancer stem cells in nasopharyngeal carcinoma. Int J Biochem Cell Biol. 2013;45:1997–2006.
Huang M, et al. Aglycone polyether nanchangmycin and its homologues exhibit apoptotic and antiproliferative activities against cancer stem cells. ACS Pharmacol Transl Sci. 2018;1:84–95.
Lu D, et al. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci USA. 2011;108:13253–7.
Ikeda H, et al. Miclxin, a Novel MIC60 inhibitor, induces apoptosis via mitochondrial stress in β-catenin mutant tumor cells. ACS Chem Biol. 2020;15:2195–204.
Kim KY, et al. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun. 2011;413:80–86.
Menke-van der Houven van Oordt CW, et al. First-in-human phase I clinical trial of RG7356, an anti-CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget. 2016;7:80046–58.
Burges A, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13:3899–905.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–32.
Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers. 2016;8:1–23.
Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling-are we there yet? Nat Rev Drug Discov. 2014;13:357–78.
Stock K, et al. Capturing tumor complexity in vitro: comparative analysis of 2D and 3D tumor models for drug discovery. Sci Rep. 2016;6:28951.
Wang H, et al. Anticancer mechanisms of salinomycin in breast cancer and its clinical applications. Front Oncol. 2021;11:654428.
Qi D, et al. Salinomycin as a potent anticancer stem cell agent: state of the art and future directions. Med Res Rev. 2022;42:1037–63.
Pádua D, et al. A SOX2 reporter system identifies gastric cancer stem-like cells sensitive to monensin. Cancers. 2020;12:495.
Tamai Y, et al. Nonactin and related compounds found in a screening program for Wnt signal inhibitory activity. Heterocylces. 2012;84:1245–50.
Shikata Y, et al. Mitochondrial uncoupler exerts a synthetic lethal effect against β-catenin mutant tumor cells. Cancer Sci. 2017;108:772–84.
Acknowledgements
We thank Shionogi & Co. Ltd for providing the three ionophores (lenoremycin sodium salt, nonactin, and nigericin sodium salt). This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H06401 (HK and MI), 19H02840 (HK), 22H04901 (HK)), and the Platform Project for Supporting Drug Discovery and Life Science Research from the Japan Agency for Medical Research and Development (AMED), Japan (HK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikeda, H., Kawami, M., Imoto, M. et al. Identification of the polyether ionophore lenoremycin through a new screening strategy for targeting cancer stem cells. J Antibiot 75, 671–678 (2022). https://doi.org/10.1038/s41429-022-00571-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-022-00571-1
Keywords
This article is cited by
-
Two new adenopeptins B and C inhibit sphere formation of pancreatic cancer cells
The Journal of Antibiotics (2024)
-
Amoxetamide A, a new anoikis inducer, produced by combined-culture of Amycolatopsis sp. and Tsukamurella pulmonis
The Journal of Antibiotics (2024)