Abstract
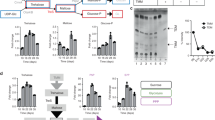
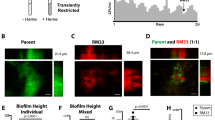
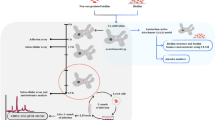
d-amino acids play an important role in cell wall peptidoglycan biosynthesis. Mycobacterium tuberculosis d-amino acid oxidase deletion led to reduced biofilm-forming ability. Other recent studies also suggest that the accumulation of d-amino acids blocks biofilm formation and could also disperse pre-formed biofilm. Biofilms are communities of bacterial cells protected by extracellular matrix and harbor drug-tolerant as well as persistent bacteria. In Mycobacterium tuberculosis, biofilm formation or its inhibition by d-amino acids is yet to be tested. In the present study, we used selected d-amino acids to study their role in the prevention of biofilm formation and also if d-cycloserine’s activity was due to presence of d-Serine as a metabolite. It was observed that d-serine limits biofilm formation in Mycobacterium tuberculosis H37Ra (Mtb-Ra), but it shows no effect on pre-formed biofilm. Also, d-cycloserine and its metabolic product, hydroxylamine, individually and in combination, with d-Serine, limit biofilm formation in Mtb-Ra and also disrupts existing biofilm. In summary, we demonstrated that d-alanine, d-valine, d-phenylalanine, d-serine, and d-threonine had no disruptive effect on pre-formed biofilm of Mtb-Ra, either individually or in combination, and d-cycloserine and its metabolite hydroxylamine have potent anti-biofilm activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
World Health Organization, 2020. Global tuberculosis report 2020. In: Global tuberculosis report. 2020.
Boshoff HI, Barry CE. Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol. 2005;3:70–80.
Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139–63.
Basaraba RJ, Ojha AK. Mycobacterial biofilms:revisiting tuberculosis bacilli in extracellular necrotizing lesions. Microbiol Spectrum. 2017;5. https://doi.org/10.1128/microbiolspec.TBTB2-0024-2016.
Islam MS, Richards JP, Ojha AK. Targeting drug tolerance in mycobacteria: a perspective from mycobacterial biofilms. Expert Rev Aanti-infective Ther. 2012;10:1055–66.
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563.
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–6.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22.
Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978;238:86–95.
Aung TT, Yam JKH, Lin S, Salleh SM, Givskov M, Liu S, Lwin NC, Yang L, Beuerman RW. Biofilms of pathogenic nontuberculous mycobacteria targeted by new therapeutic approaches. Antimicrob Agents Chemother. 2016;60:24–35.
Carter G, Wu M, Drummond DC, Bermudez LE. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J Med Microbiol. 2003;52:747–52.
Chakraborty P, Kumar A. The extracellular matrix of mycobacterial biofilms: could we shorten the treatment of mycobacterial infections? Microb Cell. 2019;6:105.
Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR Jr, Hatfull GF. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug‐tolerant bacteria. Mol Microbiol. 2008;69:164–74.
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–9.
Singh KS, Kumar R, Chauhan A, Singh N, Sharma R, Singh D, Singh SK. Knockout of MRA_1916 in Mycobacterium tuberculosis H37Ra affects its growth, biofilm formation, survival in macrophages and in mice. Tuberculosis. 2021;128:102079.
Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J Bacteriol. 2011;193:5616–22.
Aliashkevich A, Laura A, Cava F. New insights into the mechanisms and biological roles of D-amino acids in complex eco-systems. Front Microbiol. 2018;9:1–11.
Horcajo P, de Pedro MA, Cava F. Peptidoglycan plasticity in bacteria: stress-induced peptidoglycan editing by noncanonical D-amino acids. Microb Drug Resist. 2012;18:306–13.
Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009;325:1552–5.
Brandenburg KS, Rodriguez KJ, McAnulty JF, Murphy CJ, Abbott NL, Schurr MJ, Czuprynski CJ. Tryptophan inhibits biofilm formation by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57:1921–5.
Lambert MP, Neuhaus FC. Mechanism of D-cycloserine action: alanine racemase from Escherichia coli W. J Bacteriol. 1972;110:978–87.
Prosser GA, de Carvalho LPS. Kinetic mechanism and inhibition of Mycobacterium tuberculosis d‐alanine: d‐alanine ligase by the antibiotic d‐cycloserine. FEBS J. 2013;280:1150–66.
Kaushal G, Ramirez R, Alambo D, Taupradist W, Choksi K, Sirbu C. Initial characterization of D-cycloserine for future formulation development for anxiety disorders. Drug Discov Ther. 2011;5:253–60.
Olson GT, Fu M, Lau S, Rinehart KL, Silverman RB. An aromatization mechanism of inactivation of γ-aminobutyric acid aminotransferase for the antibiotic L-cycloserine. J Am Chem Soc. 1998;120:2256–67.
Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010;10:140.
Malspeis L, Gold D. Stability of cycloserine in buffered aqueous solutions. J Pharm Sci. 1964;53:1173–80.
Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol. 2014;52:4124–8.
Ghosh S, Qureshi A, Purohit HJ. D-Tryptophan governs biofilm formation rates and bacterial interaction in P. mendocina and S. aureus. J Biosci. 2019;44:3.
Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–9.
Strych U, Penland RL, Jimenez M, Krause KL, Benedik MJ. Characterization of the alanine racemases from two mycobacteria. FEMS Microbiol Lett. 2001;196:93–8.
Bruning JB, Murillo AC, Chacon O, Barletta RG, Sacchettini JC. Structure of the Mycobacterium tuberculosis D-alanine: D-alanine ligase, a target of the antituberculosis drug D-cycloserine. Antimicrob Agents Chemother. 2011;55:291–301.
Marchese A, Bozzolasco M, Gualco L, Debbia EA, Schito GC, Schito AM. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. coli biofilms. Int J Antimicrob Agents. 2003;22:95–100.
Singh KS, Sharma R, Keshari D, Singh N, Singh SK. Down-regulation of malate synthase in Mycobacterium tuberculosis H37Ra leads to reduced stress tolerance, persistence and survival in macrophages. Tuberculosis. 2017;106:73–81.
Saiman L. Clinical utility of synergy testing for multidrug-resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis: ‘the motion for’. Paediatr Respir Rev. 2007;8:249–55.
Acknowledgements
The authors would like to acknowledge funding support from SERB (Grant no. EMR/20l7/001295) and CSIR-CDRI (Grant no. MLP2033). RK is a recipient of JRF from DBT, New Delhi, India. NS, AC and MK were supported by JRF from UGC, New Delhi, India. The LC-MS/MS studies were performed at Pharmaceutics and Pharmacokinetics division of CSIR-CDRI. This manuscript is CSIR-CDRI communication no. 10404.
Author information
Authors and Affiliations
Contributions
SKS did the planning of experiments and analyzed the data. RK performed most of the experiments, prepared figures and did major writing work. NS and AC contributed to survival studies. MK and RSB contributed to LC-MS/MS studies. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kumar, R., Singh, N., Chauhan, A. et al. Mycobacterium tuberculosis survival and biofilm formation studies: effect of d-amino acids, d-cycloserine and its components. J Antibiot 75, 472–479 (2022). https://doi.org/10.1038/s41429-022-00534-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-022-00534-6