Abstract
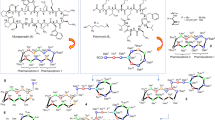
Antibiotic resistance is one of the major challenges in healthcare of our time. To meet this challenge, we designed and prepared guanidine and lipophilic guanidine derivatives of the glycopeptide antibiotic teicoplanin to armed them with activity against the most threatening nosocomial bacteria, multiresistant enterococci. From teicoplanin and its pseudoaglycone, a series of N-terminal guanidine derivatives have been prepared with free and amide C-terminal parts. Six aliphatic and aromatic lipophilic carbodiimides were prepared and used for the synthesis of lipophilic guanidine teicoplanin conjugates. All new N-terminal guanidine antibiotics showed high activity against a standard panel of Gram-positive bacteria. Four selected derivatives displayed excellent antibacterial activity against a series of nosocomial VanA Enterococcus faecium strains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Laxminarayan R. Antibiotic effectiveness: balancing conservation against innovation. Science. 2014;345:1299–301.
Cattoir V, Leclercq R. Twenty-five years of shared life with vancomycin-resistant enterococci: is it time to divorce? J Antimicrob Chemother. 2013;68:731–42.
Cattoir V, Giard J-C. Antibiotic resistance in Enterococcus faecium clinical isolates. Expert Rev Anti Infect Ther. 2014;12:239–48.
Arias CA, Murray BE. Emergence and management of drug-resistant enterococcal infections. Expert Rev Anti Infect Ther. 2008;6:637–55.
Mendes RE, et al. Longitudinal (2001-14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010-13) analysis of oritavancin in vitro potency. J Antimicrob Chemother. 2016;71:3453–9.
Sweeney D, et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. 2017;72:622–4.
Pintér G, et al. Diazo transfer-click reaction route to new, lipophilic teicoplanin and ristocetin aglycon derivatives with high antibacterial and anti-influenza virus activity: an aggregation and receptor binding study. J Med Chem. 2009;52:6053–61.
Szűcs Z, et al. Lipophilic teicoplanin pseudoaglycon derivatives are active against vancomycin- and teicoplanin-resistant enterococci. J Antibiot. 2017;70:664–70.
Szűcs Z, et al. Synthesis and biological evaluation of lipophilic teicoplanin pseudoaglycon derivatives containing a substituted triazole function. J Antibiot. 2017;70:152–7.
Szűcs Z, et al. New semisynthetic teicoplanin derivatives have comparable in vitro activity to that of oritavancin against clinical isolates of VRE. J Antibiot. 2019;72:524–34.
Vimberg V, et al. Fluorescence assay to predict activity of the glycopeptide antibiotics. J Antibiot. 2019;72:114–7.
Ishikawa T, editor. Superbases for organic synthesis. New York: Wiley; 2009.
Zamperini C, et al. Identification, synthesis and biological activity of alkyl-guanidine oligomers as potent antibacterial agents. Sci Rep. 2017;7:8251–63.
Pasero C, et al. Alkyl-guanidine compounds as potent broad-spectrum antibacterial agents: chemical library extension and biological characterization. J Med Chem. 2018;61:9162–76.
Kuppusamy R, et al. Guanidine functionalized anthranilamides as effective antibacterials with biofilm disruption activity. Org Biomol Chem. 2018;16:5877–88.
Teng P, et al. Novel bis-cyclic guanidines as potent membrane-active antibacterial agents with therapeutic potential. Chem Commun. 2017;53:11948–51.
Binda E, Marinelli F, Marcone GL. Old and new glycopeptide antibiotics: action and resistance. Antibiotics. 2014;3:572–94.
Wanner J, et al. A new and improved method for deglycosidation of glycopeptide antibiotics exemplified with vancomycin, ristocetin, and ramoplanin. Bioorg Med Chem Lett. 2003;13:1169–73.
Suhs T, König B. Synthesis of functionalized guanidino amino acids. Chem Eur J. 2006;12:8150–7.
Hosamani B, Narendra N, Prabhu G, Sureshbabu VV. Synthesis of N-urethane protected amino alkyl (S-methyl)-isothiouronium compounds and carbodiimide tethered peptidomimetics: an application for guanidino and substituted guanidino peptidomimetics synthesis. RSC Adv. 2014;4:48920–30.
Chen J, Pattarawarapan M, Zhang AJ, Burgess K. Solution- and solid-phase syntheses of substituted guanidinocarboxylic acids. J Comb Chem. 2000;2:276–81.
Fell JB, Coppola GM. A mild and efficient preparation of carbodiimides. Synth Commun. 1995;25:43–7.
Lin SW, Carver PL, DePestel DD. Dalbavancin: a new option for the treatment of gram-positive infections. Ann Pharmacother. 2006;40:449–60.
Nagarajan R. Structure-activity relationships of vancomycin-type glycopeptide antibiotics. J Antibiot. 1993;46:1181–95.
Pavlov AY, et al. Modification of glycopeptide antibiotic eremomycin by the action of alkyl halides and study on antibacterial activity of the compounds obtained. J Antibiot. 1994;47:225–32.
Malabarba A, et al. N15-alkyl and N15,N15-dialkyl derivatives of teicoplanin antibiotics. J Antibiot. 1990;43:1107–21.
Sarkar P, Yarlagadda V, Ghosh C, Haldar J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Med Chem Commun. 2017;8:516–33.
Yarlagadda V, Akkapeddi P, Manjunath GB, Haldar J. Membrane active vancomycin analogues: a strategy to combat bacterial resistance. J Med Chem. 2014;57:4558–68.
Printsevskaya SS, et al. Structure-activity relationship studies of a series of antiviral and antibacterial aglycon derivatives of the glycopeptide antibiotics vancomycin, eremomycin, and dechloroeremomycin. J Med Chem. 2005;48:3885–90.
Csávás M, et al. Synthesis and antibacterial evaluation of some teicoplanin pseudoaglycon derivatives containing alkyl- and arylthiosubstituted maleimides. J Antibiot. 2015;68:579–85.
Malabarba A, et al. Synthesis and biological properties of N63-carboxamides of teicoplanin antibiotics. Structure-activity relationships. J Med Chem. 1989;32:2450–60.
Acknowledgements
This work was supported by the European Regional Development Fund under the projects GINOP-2.3.2-15-2016-00044 and GINOP-2.3.3-15-2016-00004 and by the European Social Fund under the project EFOP-3.6.3-VEKOP-16-2017-00009. This research was also funded by the National Research, Development and Innovation Office of Hungary (K119509).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Szűcs, Z., Bereczki, I., Rőth, E. et al. N-Terminal guanidine derivatives of teicoplanin antibiotics strongly active against glycopeptide resistant Enterococcus faecium. J Antibiot 73, 603–614 (2020). https://doi.org/10.1038/s41429-020-0313-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0313-6
This article is cited by
-
Newest perspectives of glycopeptide antibiotics: biosynthetic cascades, novel derivatives, and new appealing antimicrobial applications
World Journal of Microbiology and Biotechnology (2023)
-
Synthesis of an amphiphilic vancomycin aglycone derivative inspired by polymyxins: overcoming glycopeptide resistance in Gram-positive and Gram-negative bacteria in synergy with teicoplanin in vitro
Scientific Reports (2022)
-
Semisynthetic teicoplanin derivatives with dual antimicrobial activity against SARS-CoV-2 and multiresistant bacteria
Scientific Reports (2022)