Abstract
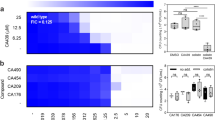
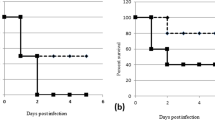
Therapeutic strategies that target bacterial virulence have received considerable attention. The type III secretion system (T3SS) is important for bacterial virulence and represents an attractive therapeutic target. Recently, we developed a new small-molecule inhibitor belonging to a class 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-ones, Fluorothiazinon (FT—previously called CL-55). FT effectively suppressed T3SS of Chlamydia spp., Pseudomonas aeruginosa, and Salmonella without affecting bacterial growth in vitro. FT was previously characterized by low toxicity, stability, and therapeutic efficacy in animal models. Salmonella T3SS inhibition by FT was studied using in vitro assays for effector proteins detection and estimation of salmonella replication in peritoneal macrophages. The antibacterial effect of FT in vivo was investigated in murine models of salmonella chronic systemic and acute infection. Oral administration of the virulent strain of Salmonella enterica serovar Typhimurium to mice-induced chronic systemic infection with the pathogen persistence in different lymphoid organs such as spleens, Peyer’s plaques, and mesenteric lymph nodes. We found that FT suppressed orally induced salmonella infection both with therapeutic and prophylactic administration. Treatment by FT at a dose of 50 mg/kg for 4 days starting from day 7 post-infection (therapy) as well as for 4 days before infection (prevention) led to practically complete eradication of salmonella in mice. FT shows a strong potential for antibacterial therapy and could be used as a substance in the design of antibacterial drugs for pharmaceutical intervention including therapy of antibiotic-resistant infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Qianghua LV, et al. Inhibition of the type III secretion system by syringaldehyde protects mice from Salmonella enterica serovar Typhimurium. J Cell Mol Med. 2019;23:4679–88.
Gal-Mor O. Persistent infection and long-term carriage of typhoidal and nontyphoidal Salmonellae. Clin Microbiol Rev. 2019;32:1–31.
Knodler LA. Salmonella enterica: living a double life in epithelial cells. Curr Opin Microbiol. 2015;23:23–31.
WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics 2017. https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
Czaplewski L, et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect Dis. 2016;16:239–51.
Munguia J, Nizet V. Pharmacological targeting of the host-pathogen interaction: alternatives to classical antibiotics to combat drug-resistant superbugs. Trends Pharm Sci. 2017;38:473–88.
Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol. 2014;12:300–8.
Theuretzbacher U, Piddock LJV. Non-traditional antibacterial therapeutic options and challenges. Cell Host Microbe. 2019;26:61–72.
McShan AC, De, Guzman RN. The bacterial type III secretion system as a target for developing new antibiotics. Chem Biol Drug Des. 2015;85:30–42.
Fasciano AC, Shaban L, Mecsas J. Promises and challenges of the type three secretion system injectisome as an antivirulence target. In: Sandkvist M, Cascales E, Christie PJ, editors. Protein Secretion in Bacteria. Chapter 21. Wiley Online Library: https://doi.org/10.1128/9781683670285. 2019; p. 261–276
Charro N, Mota LJ. Approaches targeting the type III secretion system to treat or prevent bacterial infections. Expert Opin Drug Discov. 2015;10:373–87.
Duncan MC, Linington RG, Auerbuch V. Chemical inhibitors of the type three secretion system: disarming bacterial pathogens. Antimicrob Agents Chemother. 2012;56:5433–41.
Zhang Y, Liu Y, Wang T, Deng X, Chu X. Natural compound sanguinarine chloride targets the type III secretion system of Salmonella enterica Serovar Typhimurium. Biochem Biophys Rep. 2018;14:149–54.
Nesterenko LN, et al. A small-molecule compound belonging to a class of 2,4-disubstituted 1,3,4-thiadiazine-5-ones suppresses Salmonella infection in vivo. J Antibiot. 2016;69:422–7.
Zigangirova NA, et al. A small-molecule compound belonging to a class of 2,4-disubstituted 1,3,4-thiadiazine-5-ones inhibits intracellular growth and persistence of Chlamydia trachomatis. J Med Microbiol. 2016;65:91–8.
Koroleva EA, et al. Small molecule inhibitor of type three secretion suppresses acute and chronic Chlamydia trachomatis infection in a novel urogenital Chlamydia model. Biomed Res Int. 2015;2015:484853.
Zigangirova NA, et al. Development of Chlamydial type III secretion system inhibitors for suppression of acute and chronic forms of Chlamydial infection. Acta Nat. 2012;4:87–97.
Sheremet AB, et al. Small molecule inhibitor of type three secretion system belonging to a class 2,4-disubstituted-4h-[1,3,4]-thiadiazine-5-ones improves survival and decreases bacterial loads in an airway Pseudomonas aeruginosa infection in mice. Biomed Res Int. 2018;2018:5810767.
Negrea A, et al. Salicylidene acylhydrazides that affect type III protein secretion in Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother. 2007;51:2867–76.
Nesterenko LN, et al. Mycobacterium tuberculosis-susceptible I/St mice develop severe disease following infection with taxonomically distant bacteria, Salmonella enterica and Chlamydia pneumoniae. Clin Exp Immunol. 2006;146:93–100.
Figueira R, Watson KG, Holden DW, Helaine S. Identification of salmonella pathogenicity island-2 type III secretion system effectors involved in intramacrophage replication of S. enterica serovar Typhimurium: implications for rational vaccine design. mBio. 2013;4:e00065.
Kobets NV, Nesterenko LN, Balunets DV. Local and systemic immune responses to Salmonella in genetically susceptible I/St mice after mucosal challenge. ISRN Immunol. 2013;2013:7.
Zha L, Garrett S, Sun J. Salmonella infection in chronic inflammation and gastrointestinal cancer. Diseases. 2019;7:1–14.
Palmer AD, Slauch JM. Mechanisms of Salmonella pathogenesis in animal models. Hum Ecol Risk Assess. 2017;23:1877–92.
Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J Exp Med. 2004;199:231–41.
Gunn JS, et al. Salmonella chronic carriage: epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014;22:648–55.
Karatzas KA, et al. Phenotypic and proteomic characterization of multiply antibiotic-resistant variants of Salmonella enterica serovar Typhimurium selected following exposure to disinfectants. Appl Environ Microbiol. 2008;74:1508–16.
Wang M, Qazi IH, Wang L, Zhou G, Han H. Salmonella virulence and immune escape. Microorganisms. 2020;8:1–25.
Buckner MM, Croxen MA, Arena ET, Finlay BB. A comprehensive study of the contribution of Salmonella enterica serovar Typhimurium SPI2 effectors to bacterial colonization, survival, and replication in typhoid fever, macrophage, and epithelial cell infection models. Virulence 2011;2:208–16.
Jennings E, Thurston TLM, Holden DW. Salmonella SPI-2 type iii secretion system effectors: molecular mechanisms and physiological consequences. Cell Host Microbe. 2017;22:217–31.
Figueira R, Holden DW. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology. 2012;158(Pt 5):1147–61.
Mastroeni P, Grant AJ. Spread of Salmonella enterica in the body during systemic infection: unravelling host and pathogen determinants. Expert Rev Mol Med. 2011;13:e12.
Anantharajah A, Mingeot-Leclercq MP, Van Bambeke F. Targeting the type three secretion system in Pseudomonas aeruginosa. Trends Pharm Sci. 2016;37:734–49.
Anantharajah A, et al. Salicylidene acylhydrazides and hydroxyquinolines act as inhibitors of type three secretion systems in Pseudomonas aeruginosa by distinct mechanisms. Antimicrob Agents Chemother. 2017;61:1–17.
De Tavernier E, et al. High throughput combinatorial formatting of PcrV nanobodies for efficient potency improvement. J Biol Chem. 2016;291:15243–55.
Thanabalasuriar A, et al. Bispecific antibody targets multiple Pseudomonas aeruginosa evasion mechanisms in the lung vasculature. J Clin Investig. 2017;127:2249–61.
Le HN, et al. Treatment efficacy of MEDI3902 in Pseudomonas aeruginosa bloodstream infection and acute pneumonia rabbit models. Antimicrob Agents Chemother. 2019;63:5.
Anantharajah A, et al. Inhibition of the injectisome and flagellar type iii secretion systems by INP1855 impairs Pseudomonas aeruginosa pathogenicity and inflammasome activation. J Infect Dis. 2016;214:1105–16.
McHugh RE, O’Boyle N, Connolly JPR, Hoskisson PA, Roe AJ. Characterization of the mode of action of aurodox, a type iii secretion system inhibitor from Streptomyces goldiniensis. Infect Immun. 2019;87:12.
Kimura K, et al. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J Antibiot. 2011;64:197–203.
Acknowledgements
This work was supported by grants from the Russian Ministry of Science and Education and the Russian Ministry of Industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zigangirova, N.A., Nesterenko, L.N., Sheremet, A.B. et al. Fluorothiazinon, a small-molecular inhibitor of T3SS, suppresses salmonella oral infection in mice. J Antibiot 74, 244–254 (2021). https://doi.org/10.1038/s41429-020-00396-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-00396-w
This article is cited by
-
Pharmacokinetics, tissue distribution, bioavailability and excretion of the anti-virulence drug Fluorothiazinon in rats and rabbits
The Journal of Antibiotics (2024)
-
Development and Validation of a Quantitative Determination Method for Fluorothiazinone in Human Blood Plasma
Pharmaceutical Chemistry Journal (2024)
-
Using next generation antimicrobials to target the mechanisms of infection
npj Antimicrobials and Resistance (2023)
-
Antibiotics in the clinical pipeline as of December 2022
The Journal of Antibiotics (2023)
-
Fluorothiazinon inhibits the virulence factors of uropathogenic Escherichia coli involved in the development of urinary tract infection
The Journal of Antibiotics (2023)