Abstract
Exploration of microbial dynamics of Streptomyces lavendulae ACR-DA1, a psychrotrophic isolate from the North-Western Himalayan cold desert, was carried out using matrix-assisted laser desorbtion ionisation-time of flight mass spectrometer. Valinomycin was found as a major produce and cyclic depsipeptide montanastatin as a minor produce. The yield of the valinomycin was found to be 0.3 mg l−1 in submerged growth condition at the batch scale. Miniaturization of optimization experiments was adept to maximize the production using the expeditious and efficient technique of intact cell mass spectrometry. The present study showed that using optimized conditions and growing the culture in synthetic mineral base starch medium at 10 °C enhanced the production to 19.4 mg l−1. Our results demonstrated 64-fold increase in yield from the wild-type S. lavendulae ACR-DA1 strain using a simple and economical downstream process.
Similar content being viewed by others
Introduction
Microorganisms, blessed with enormous diversity encapsulating plethora of secondary metabolites possess the potential for drug discovery either as therapeutic agents, or for agricultural applications and industrial use. Secondary metabolites comprising of peptide natural products get assembled distinctly either by ribosomal or by non-ribosomal route occurring in a wide range of microorganisms including fungi and bacteria with unique metabolic capabilities [1]. Being geographically located closely to the North-Western Himalayan Region (NWHR) endowed with microbial biodiversity, we carried out the bio-prospection of microorganisms from every possible resource of this unique niche including extreme environments such as cold deserts and habitats [2].
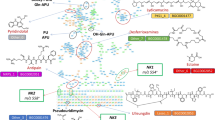
The strain Streptomyces lavendulae ACR-DA1, an isolate of poorly explored Drass, Kargil, the region in India of Himalayan cold deserts with temperatures ranging from (−) 45 °C to 15 °C at 10,760 feet above the sea level, was explored for its microbial dynamics. Observation by mass studies using rapid and efficient intact cell mass spectrometry (ICMS) technique by MS, showed its rich valine content. The complete characterization using chromatographic and spectrometric techniques confirmed it as valinomycin [3,4,5]. The cyclododecadepsipeptide has been reported for its antimicrobial [6], antifungal [7], antiviral [8], anticancer [9], and antineoplastic activities [9] with three repeating units of d-hydroxyisovaleryl (d-Hiv), -d-Valyl (d-Val), -l-Lactyl (l-Lac) and -l-Valyl (l-Val). Another cyclic depsipeptide montanastatin [9, 10], with two repeating units of the same monomer, has also been found to be produced from this psychotropic strain (Fig. 1).
Majority of microbial producers of non-ribosomal peptides are either not cultivable or low in production yields paving difficulties in carrying out studies for isolation of secondary metabolites. Optimization of growth conditions becomes impecunious for enhanced production of desirable metabolites from microbes [11, 12]. To the best of our knowledge, no optimization study has been reported on the wild-type strain for the isolation of valinomycin. However, scale-up of non-ribosomal antibiotic peptide valinomycin from Streptomyces tsusimaensis had been recombinantly done in Escherichia coli, improving its titer from 0.5 to 3.3 mg l−1 and increasing it to 13 mg l−1 with repetitive feeding of a glucose polymer during cultivation. Yield of valinomycin has also been significantly improved from 0.3 to 2.4 mg l−1 by switching from batch to enzyme based fed batch mode in the shake flask condition and the repetitive glucose polymer feed to enzyme based fed batch mode which further increased its titer to 10 mg l−1 [13, 14]. In our previous work, we have shown in dichloromethane extract that agitation under low temperature conditions in MBS medium resulted in overproduction of valinomycin synthetase genes vlm1 and vlm2 [15] as observed by its titers using LC-MS/MS. The studies were further carried out for isolation and optimization of culture conditions for production of bioactive valinomycin for its enhanced production.
Results and discussion
Microorganism, culture, and growth conditions
The psychrotrophic actinomycete strain ACR-DA1 used in the present study was isolated from the cold deserts of NWHR as described previously [15]. The culture grows well over the entire temperature range from 4 °C to 37 °C covering psychrophilic to mesophilic range (Fig. S1, Supplementary Information) and established itself as a psychrotrophic actinobacterium with the potential to survive and sustain at a wide temperature range.
Identification of depsipeptides
The preliminary examination conducted through mass spectroscopic analysis showed mass with m/z 741.4277 (ESI-QTOF MS) (Fig. 2a) and m/z 1133.7, 1149.6 (MALDI-TOF MS) (Fig. 2b). The observation by MS/MS studies of m/z 741.4277 showed fragment ion as m/z 642.3608, 542.3406, 443.2332, 343.2226 and revealed the cyclic structure as montanastatin (Fig. S2, Supplementary Information). The fragmentation studies carried out for m/z 1133.7 [M + Na]+ revealed high content of valine with close resemblance to a cyclic structure (Fig. S3, Supplementary Information). The characterization done using chromatographic and spectrometric studies by NMR and MS established the structure as valinomycin (Fig. 3a–c). A comparison was made with the standard valinomycin procured from Sigma Aldrich and we found that spectra and chromatograms were in complete corroboration with the assignment of isolated compound as valinomycin (Figs. S4–S8, Supplementary Information). The biological activity of potassium ionophoric non-ribosomal cyclododecadepsipeptide valinomycin was evaluated against Mycobacterium tuberculosis H37Rv and cytotoxic activity against a panel of cancer cell lines along with a comparative study done with procured Sigma–Aldrich standard (Tables S1 and S2, Supporting Information).
The symmetric 36-membered ring valinomycin was found to be the major produce of psychrotrophic actinomycete strain S. lavenduale isolated from NHWR and showed production of 0.3 mg l−1 in a shake flask using dextrin peptone broth (DPB) medium. Because of its biological significance, studies for optimization of growth conditions were taken up for its enhanced production.
Effect of growth conditions on valinomycin production
Studies on varied temperature range supported selective production of valinomycin at 4 °C, but it was found to be less compared to the production obtained at 10 °C as shown in Fig. S9, Supplementary Information. At higher temperatures, production of other metabolites was observed but as our focus lied on selective and maximized production of valinomycin, so 10 °C was chosen as more preferred temperature (Table 1, Fig. S9, Supplementary Information). Inference of spectral data carried out on varied pH range indicated optimum production in neutral to slightly basic condition (Table 1, Fig. S10, Supplementary Information). No significant light effect was observed on the growth conditions of actinomycete strain (Table 1, Fig. S11, Supplementary Information). Herein, in all the experiments the culture was incubated for a period of 15 days and evaluated for valinomycin production.
In submerged phase, studies were conducted using the optimized conditions obtained under solid phase experiments viz. 10 °C temperature and 7.0 pH in both static and shaking conditions. Production of valinomycin almost gets doubled in a shake flask when compared to that in static conditions (Table 1, Figs. S12 and S13, Supplementary Information). Based on the observation from above experiments, further studies were then carried out only in the shake flask submerged growth condition. Variable temperature and pH studies were carried out in a shake flask in submerged phase and results were found to be in corroboration. The maximum production of valinomycin in submerged phase was obtained at pH of 7.0 and temperature of 10 °C giving the isolated yield of 0.3 mg l−1 (Figs. S14 and S15, Supplementary Information). Other varied optimization experiments like volume to air ratio, % of inoculums, rpm, and culture media were also carried out in shake flask submerged conditions. It was inferred that 1:9 volume to air ratio, 1.5% of inoculum using 200 rpm was found to be optimal for the growth of valinomycin as shown in supporting information (Figs. S16–S20, and Table S3 Supplementary Information). On the basis of above optimization experiments, fermentation studies were then further carried out for large scale production at the fermenter level.
Fermentation studies in varied type of fermenters used for study showed bubble column and stirred tank supporting appreciable quantity of valinomycin production (Table 1). The fermentation conditions used for this study were based on finding of our previous study, which showed that the encoded components of heterodimer valinomycin synthetase genes vlm1 and vlm2 get over expressed at low temperatures and in mineral base starch (MBS) medium [15]. The experiments were analyzed by using RT-PCR and reported titers in dichloromethane extract using LC-MS/MS. Experiments were carried out in a stirred tank fermenter at 5 l scale using optimized condition at 10 °C, 0.5 vvm air having agitation speed of 200 rpm in DPB (control) and in MBS (synthetic) medium (Table 1, Figs. S21–S23, Supplementary Information).
A study was done on extract obtained from cell mass and broth of DPB and MBS medium using high performance thin layer chromatography (HPTLC) using solvent system 30% ethyl acetate in hexane with 1% formic acid. Derivatization reagent used was anisaldehyde sulfuric acid. It was found that growing the valinomycin in MBS medium, the production was mainly obtained from cell mass, whereas in DPB medium it was mainly obtained from broth as shown in Fig. 4. In DPB the yield was 2.3 mg l−1 as 10.2 mg of valinomycin was obtained from 4.5 l of fermented broth. Whereas using synthetic MBS medium with polymeric starch as the sole carbon source, 87.5 mg of valinomycin was obtained from 4.5 l of fermented broth giving the yield of 19.4 mg l−1. These studies directed us that the MBS medium was a preferable medium for production and found to be the more economical both from production and downstream perspective. Valinomycin production increased during the first 4–8 days of incubation and attained stationary phase with the highest production at eighth day (Table 1, Fig. S23, Supplementary Information). Hereby, in present study we report the enhanced yield of valinomycin with 64-fold increase having 99.8% purity (Fig. S4, Supplementary Information). Confirmation of valinomycin was done by LC-MS (Fig. S6, Supplementary Information).
Purification of depsipeptides
Valinomycin obtained at retention time ranging between 55.6 and 61.1 min as fraction F16 (Fig. S24, Supplementary Information). The isolated yield was found to have 99.8% purity. The minor fraction F14 was obtained at retention time tR at 41.6 min (Fig. S24, Supplementary Information). The mass studies showed m/z 741.4277 [M + H]+ as observed using HR-ESIQ-TOFMS calculated for C36H41N4O12. The fragmentation study of m/z 741.4277 [M + H]+ confirmed the structure as montanastatin shown in Fig. 2a, which was found as minor produce from this actinomycete isolate of NWHR.
Isolation and optimization studies were conducted for the enhanced production of valinomycin from wild-type S. lavendulae, ACR-DA1 from the North-Western Himalayas. Valinomycin was isolated as major product (87.5 mg) and montanastatin as minor product (0.4 mg). Our studies emphasize optimization as crucial for selective and maximized production of desirable secondary metabolites. Several research studies have revealed that different growth fermentation factors and environmental conditions play good tricks with the microbial dynamics indicating optimization as crucial necessity for enhanced production of secondary metabolites, which many times gets bottlenecked due to trace levels of production [16, 17]. Our studies have shown that production of valinomycin in isolation media (DPB medium) was only 0.3 mg l−1. After the strategic optimization from batch scale to fermenter level, the production was enhanced up to 19.4 mg l−1. Factors found crucial for enhanced production of valinomycin were pH, temperature and culture media. Using the synthetic MBS medium at 10 °C enhanced the selective production by 64-fold from the wild-type S. Lavendulae ACR-DA1. Thus, the optimized conditions led to increased production from 0.3 mg l−1 to 19.4 mg l−1. Isolation of valinomycin from the psychrotrophic isolate S. lavendulae of North-Western Himalayas provided an easy and cost-effective downstream process in comparison to the bulk requirements of organic solvents, which largely remains a rate-limiting factor and major bottlenecks both from resource and time scale perspectives. Enhanced yield of 19.4 mg l−1 was achieved as final outcome of present optimization study and was found comparable with mesophiles viz. Streptomyces exfoliatus (32.8 mg l−1), Streptomyces anulatus (25.22 mg l−1), Streptomyces griseus (22.08 mg l−1), and Streptomyces sp. PRL1642 (23.19 mg l−1) [18] which reported their titers but not isolated yields.
Experimental
General experimental procedures
All the chemicals, medium components, and reagents were bought from Himedia, Sigma, Merck and Fischer scientific, respectively. Valinomycin was procured from Sigma Aldrich. Analytical HPLC was carried out on Shimadzu HPLC system and semi-preparative HPLC purification was carried out on ThermoFinnigan HPLC system connected with quaternary pump and photodiode array detector using reversed-phase (Merck, LiChrospher) RP18e, 125 × 4 mm, 5 µm column for analytical and (Dr. Maisch GmbH, Reprosil Gold) C18, 10 × 250 mm, 5 µm column for semi-preparative separation. Mass analysis was carried out on an ABSciex 4800 MALDI-TOF/TOF instrument. LC-MS was carried out on WATERS Quattro Premier Micromass with Alliance system and HRMS was carried on Agilent 6540 UHD Q-TOF coupled with Aglient 1290 infinity series HPLC.
Production medium
Cultivation medium used to isolate and maintain S. lavendulae ACR-DA1 was dextrin peptone agar with constituents: Dextrin 1.5%, peptone 0.5%, yeast extract 0.5%, K2HPO4 0.5%, NaH2PO4 0.06%, MgSO4 0.05% and agar 2.0%, with pH adjusted to 7.0. The seed medium was prepared by inoculating loop full of culture from a freshly cultured slant to 100 ml DPB medium in 250-ml Erlenmeyer flask. Incubation was carried out for 3 days at 28 °C in an orbital shaker at 200 rpm. There were two stages of preparation, the first stage was from a slant to the flask where 1% v/v of the seed medium was used, and in the second stage 1.5% v/v of the seed medium was used from the flask for the optimization at fermenter level. The composition of MBS medium is as follows: Starch 1.0%, CaCO3 0.3%, K2HPO4 0.1%, (NH4)2SO4 0.2%, MgSO4 0.1% and NaCl 0.1% with pH adjusted to 7.0 [15].
ICMS procedure using MALDI-TOF MS
To get insight into secondary metabolite production from the actinomycete strain cell mass was carefully harvested from a petri plate and macerated using methanol and subjected to (ICMS) intact-cell mass spectrometric study using MALDI-TOF mass spectrometer. The matrix used was CHCA (α-cyano-4-hydroxy cinnamic acid) in the concentration of 10 mg ml−1 in acetonitrile: water in the ratio of 70:30 by volume. Preparation of homogenous sample was ensured by vertexing for 10 min followed by centrifugation at 5000 rpm for 10 min. Supernatant was collected and mixed with the matrix in varied ratio of 1:1, 2:1, 5:1 or 25:1. One microlitre of well homogenized solution was spotted onto target wells of a 96 or 364 well sample plate and air dried prior to analysis.
Optimization study
Studies were conducted at both solid phase (petri plate level) and submerged phase. Different experiments were carried out with minimum of three-time repeatability. Solid phase studies were conducted with varying temperature, pH, and light effects. To monitor temperature effect, a study was conducted at 4 °C, 10 °C, 20 °C, 30 °C, 37 °C, and 45 °C keeping the pH at 7.0 and pH study was carried out at the variable range from 6.0 to 9.0. Light effect was monitored by growing the culture simultaneously in light and dark conditions (Figs. S9–S11, Supplementary Information). In submerged phase, studies were conducted in static and shaking conditions under optimized conditions as obtained in solid state studies (Figs. S12 and S13, Supplementary Information). Since better production was observed in submerged shaking condition therefore various optimization experiments were conducted at submerged shaking condition, which were in concurrence with the solid phase studies (Figs. S14–S20, Supplementary Information). Further scale-up studies were conducted in different bioreactors using optimized fermentation conditions (Figs. S21–S23, Supplementary Information).
Bioreactors used
For the optimal production of valinomycin, three different bioreactors were employed for batch fermentation by inoculating with 1.5% of 3 days old seed culture of ACR-DA1 at 30 °C and then reducing the temperature gradually to 10 °C. Fermentation was carried out for a period of 8 days at 10 °C with the help of chiller/circulator (Haake K 10, Thermo electron Corporation). Stirred tank fermenter (7 l, Bioflow) with working volume of 5 l was operated at agitation of 200 rpm and with 0.5 vvm air. Bubble Column fermenter (3 l, Bioengineering) was sparged with 0.5vvm air. No additional source of agitation was employed. Air Lift fermenter (5 l, Bioengineering) was sparged with 0.5 vvm air with same air pressure as for Bubble Column.
Purification of depsipeptides
For purification the crude organic extract was dissolved in methanol and centrifuged. Supernatant was loaded on Sephadex LH 20 resin and eluted with methanol. Fractions collected were evaporated and examined for valinomycin production. Pooled fractions were evaporated to dryness under vacuum to obtain semi-purified peptide mixture. The resulting extracts were further purified through RP-HPLC using 10 × 250 mm, C18 column (Dr. MaischGmbH, Reprosil Gold, 5 µm) with water/methanol as eluent under gradient condition with gradual increase from 30 to 95% methanol in 15 min, then keeping 95% methanol for next 45 min followed by decrease in concentration from 95 to 30% in 5 min, at the flow rate of 1.5 ml min−1 and detection at 214 nm.
References
Hegemann JD, Zimmermann M, Xie X, Marahiel MA. Lasso peptides: an intriguing class of bacterial natural products. Acc Chem Res. 2015;48:1909–19.
Singh VP, et al. Lipovelutibols A-D: cytotoxic lipopeptaibols from the Himalayan cold habitat fungus Trichoderma velutinum. J Nat Prod. 2018;81:219–26.
Brockmann H, Kastner GS. Valinomycin I, XXVII. Msg. about antibiotics from actinomycetes. Chem Ber. 1955;88:57–61.
Goodwin CR, Fenn LS, Derewacz DK, Bachmann BO. Structural mass spectrometry: rapid methods for separation and analysis of peptide natural products. J Nat Prod. 2012;75:48–53.
Sheil MM, Kilby GW, Curtis JM, Bradley CD, Derrick PJ. Low‐energy tandem mass spectra of the cyclic depsipeptide valinomycin - a comparison with four‐sector tandem mass spectra. Org Mass Spectrom. 1993;28:574–6.
Tempelaars MH, Rodrigues S, Abee T. Comparative analysis of antimicrobial activities of valinomycin and cereulide, the Bacillus cereus emetic toxin. Appl Environ Microbiol. 2011;77:2755–62.
Park CN, Lee JM, Lee D, Kim BS. Antifungal activity of valinomycin, a peptide antibiotic produced by Streptomyces sp. strain M10 antagonistic to Botrytis cinerea. J Microbiol Biotechnol. 2008;18:880–4.
Wu CY, et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci USA. 2004;101:10012–7.
Pettit GR, et al. Antineoplastic agents. Part 409: Isolation and structure of montanastatin from a terrestrial actinomycete. Bioorg Med Chem. 1999;7:895–9.
Doi M, Asano A. Montanastatin, cyclo[-(Val-D-Hyv-D-Val-Lac)2−]. Acta Cryst. 2002;E58:o935–o936.
Bundale S, Begde D, Nashikkar N, Kadam T, Upadhyay A. Optimization of culture conditions for production of bioactive metabolites by Streptomyces spp. isolated from soil. Adv Microbiol. 2015;5:441–51.
da Silva IR, Martins MK, Carvalho CM, de Azevedo JL, de Lima, Procópio RE. The effect of varying culture conditions on the production of antibiotics by Streptomyces spp., isolated from the Amazonian soil. Ferment Technol. 2012;1:105.
Li J, Jaitzig J, Hillig F, Sussmuth R, Neubauer P. Enhanced production of the nonribosomal peptide antibiotic valinomycin in Escherichia coli through small-scale high cell density fed-batch cultivation. Appl Microbiol Biotechnol. 2014;98:591–601.
Li J, et al. Type II thioesterase improves heterologous biosynthesis of valinomycin in Escherichia coli. J Biotechnol. 2015;193:16–22.
Sharma R, et al. Revelation and cloning of valinomycin synthetase genes in Streptomyces lavendulae ACR-DA1 and their expression analysis under different fermentation and elicitation conditions. J Biotechnol. 2017;253:40–47.
Bode HB, Bethe B, Hofs R, Zeeck A. Big effects from small changes: possible ways to explore nature’s chemical diversity. Chem Bio Chem. 2002;3:619–27.
Wu C, Choi YH, van Wezel GP. Metabolic profiling as a tool for prioritizing antimicrobial compounds. J Ind Microbiol Biotechnol. 2016;43:299–312.
Matter AM, Hoot SB, Anderson PD, Neves SS, Cheng YQ. Valinomycin biosynthetic gene cluster in Streptomyces: conservation, ecology and evolution. PLoS ONE. 2009;4:e7194.
Acknowledgements
We express our sincere thanks to Er. Rajneesh Anand for his support in Instrumentation Division. We are indebted to Dr. Inshad Ali Khan, Dr. Shashi Bhushan and their teams for carrying out biological activity. VPS and RS thank CSIR for Research Fellowship. The current work was supported by the funding received from CSIR Mega Lab Project MLP 4010, 4014 and networking project BSC 0117 (Plant-Microbe and Soil Interactions). This manuscript bears institutional publication number IIIM/2153/2017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Singh, V.P., Sharma, R., Sharma, V. et al. Isolation of depsipeptides and optimization for enhanced production of valinomycin from the North-Western Himalayan cold desert strain Streptomyces lavendulae. J Antibiot 72, 617–624 (2019). https://doi.org/10.1038/s41429-019-0183-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0183-y