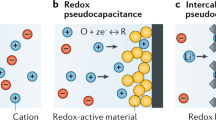
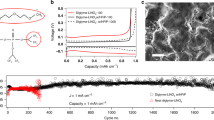
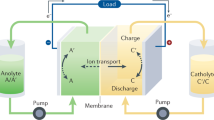
Abstract
Redox-active polymers with charging/discharging reversibility are employed to develop electrode-active materials in organic batteries, which are characterized by high power rates, flexibility/bendability, and environmentally benign properties. Reversible charge storage with polymers is achieved by redox “bistability” and exchange reactions. Redox bistability is a feature of electrochemical reversibility, which refers to the properties of redox pairs in which both the reduced and oxidized states are chemically robust and do not fade during substantial storage periods. The electron self-exchange reactions of the redox-active sites populated in the polymer layer give rise to charge propagation in support of exhaustive charging and discharging. The concept of charge storage reversibility is extended to hydrogen storage reversibility based on the bistability of the hydrogenation/dehydrogenation pair and the electron/proton exchange reaction, creating hydrogen carrier polymers as a new class of energy-related functional polymers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Goujon N, Casado N, Patil N, Marcilla R, Mecerreyes D. Organic batteries based on just redox polymers. Prog Polym Sci. 2021;122:101449.
Gracia R, Mecerreyes D. Polymers with redox properties: materials for batteries, biosensors and more. Polym Chem. 2013;4:2206–14.
Zhao Q, Zhu Z, Chen J. Molecular engineering with organic carbonyl electrode materials for advanced stationary and redox flow rechargeable batteries. Adv Mater. 2017;29:1607007.
Poizot P, Gaubicher J, Renault S, Dubois L, Liang Y, Yao Y. Opportunities and challenges for organic electrodes in electrochemical energy storage. Chem Rev. 2020;120:6490–6557.
Kim J, Kim Y, Yoo J, Kwon G, Ko Y, Kang K. Organic batteries for a greener rechargeable world. Nat Rev Mater. 2023;8:54–70.
Shea JJ, Luo C. Organic electrode materials for metal ion batteries. ACS Appl Mater Interfaces. 2020;12:5361–80.
Han C, Li H, Shi R, Zhang T, Tong J, Lia J, et al. Organic quinones towards advanced electrochemical energy storage: recent advances and challenges. J Mater Chem A. 2019;7:23378–415.
Esser B, Dolhem F, Becuwe M, Poizot P, Vlad A, Brandell D. A perspective on organic electrode materials and technologies for next generation batteries. J Power Sour. 2021;482:228814.
Poizot P, Dolhem F, Gaubicher J. Progress in all-organic rechargeable batteries using cationic and anionic configurations: toward low-cost and greener storage solutions? Curr Opin Electrochem. 2018;9:70–80.
Wu Y, Zeng R, Nan J, Shu D, Qiu Y, Chou SL. Quinone electrode materials for rechargeable lithium/sodium ion batteries. Adv Energy Mater. 2017;7:1700278.
Gan X, Yang Z, Song Z. Solid-state batteries based on organic cathode materials. Batteries Supercaps 2023;6:e202300001.
Song ZP, Zhou HS. Towards sustainable and versatile energy storage devices: an overview of organic electrode materials. Energy Environ Sci. 2013;6:2280–301.
Wang H, Wu Q, Cheng L, Zhu G. The emerging aqueous zinc-organic battery. Coord Chem Rev. 2022;472:214772.
MacInnes Jr. D, Druy MA, Nigrey PJ, Nairns DP, MacDiarmid AG, Heeger AJ. Organic batteries: reversible n- and p- type electrochemical doping of polyacetylene, (CH)x. J Chem Soc Chem Commun. 1981; 317–19.
Mike JF, Lutkenhaus JL. Electrochemically active polymers for electrochemical energy storage: opportunities and challenges. ACS Macro Lett. 2013;2:839–44.
Mike JF, Lutkenhaus JL. Recent advances in conjugated polymer energy storage. J Polym Sci B. 2013;51:468–80.
Novák P, Müller K, Santhanam KSV, Haas O. Electrochemically active polymers for rechargeable batteries. Chem Rev. 1997;97:207–81.
Nishide H. Organic redox polymers as electrochemical energy materials. Green Chem. 2022;24:4650–79.
Wang S, Easley AD, Lutkenhaus JL. 100th Anniversary of macromolecular science viewpoint: fundamentals for the future of macromolecular nitroxide radicals. ACS Macro Lett. 2020;9:358–70.
Janoschka T, Hager MD, Schubert US. Powering up the future: radical polymers for battery applications. Adv Mater. 2012;24:6397–409.
Tomlinson EP, Hay ME, Boudouris BW. Radical polymers and their application to organic electronic devices. Macromolecules. 2014;47:6145–58.
Hatakeyama-Sato K, Oyaizu K. Redox: organic robust radicals and their polymers for energy conversion/storage devices. Chem Rev. 2023;123:11336–91.
Nishide H, Oyaizu K. Toward flexible batteries. Science. 2008;319:737–38.
Oyaizu K, Nishide H. Radical polymers for organic electronics: a radical departure from conjugated polymers? Adv Mater. 2009;21:2339–44.
Nakahara K, Oyaizu K, Nishide H. Organic radical battery approaching practical use. Chem Lett. 2011;40:222–7.
Xie Y, Zhang K, Yamauchi Y, Oyaizu K, Jia Z. Nitroxide radical polymers for emerging plastic energy storage and organic electronics: fundamentals, materials, and applications. Mater Horiz. 2021;8:803–29.
Oyaizu K, Nishide H. Macromolecular complexes leading to high performance energy devices. Macromol Symp. 2012;317-318:248–58.
Suga T, Pu YJ, Oyaizu K, Nishide H. Electron-transfer kinetics of nitroxide radicals as an electrode-active material. Bull Chem Soc Jpn. 2004;77:2203–4.
Koshika K, Sano N, Oyaizu K, Nishide H. An ultrafast chargeable polymer electrode based on the combination of nitroxide radical and aqueous electrolyte. Chem Commun. 2009; 836–38.
Koshika K, Sano N, Oyaizu K, Nishide H. An aqueous electrolyte-type rechargeable device utilizing a hydrophilic radical polymer-cathode. Macromol Chem Phys. 2009;210:1989–95.
Koshika K, Chikushi N, Sano N, Oyaizu K, Nishide H. A TEMPO-substituted polyacrylamide as a new cathode material: an organic rechargeable device composed of polymer electrodes and aqueous electrolyte. Green Chem. 2010;12:1573–75.
Zhuang X, Xiao C, Oyaizu K, Chikushi N, Chen X, Nishide H. Synthesis of amphiphilic block copolymers bearing stable nitroxyl radicals. J Polym Sci A. 2010;48:5404–10.
Sano N, Tomita W, Hara S, Min CH, Lee JS, Oyaizu K, et al. Polyviologen hydrogel with high-rate capability for anodes toward an aqueous electrolyte-type and organic-based rechargeable device. ACS Appl Mater Interfaces. 2013;5:1355–61.
Sato K, Katagiri R, Chikushi N, Lee S, Oyaizu K, Lee JS, et al. Totally organic-based bendable rechargeable devices composed of hydrophilic redox polymers and aqueous electrolyte. Chem Lett. 2017;46:693–94.
Nishide H, Koshika K, Oyaizu K. Environmentally benign batteries based on organic radical polymers. Pure Appl Chem. 2009;81:1961–70.
Koshika K, Kitajima M, Oyaizu K, Nishide H. A rechargeable battery based on hydrophilic radical polymer-electrode and its green assessment. Green Chem Lett Rev. 2009;2:169–74.
Zhuang X, Zhang H, Chikushi N, Zhao C, Oyaizu K, Chen X, et al. Biodegradable and electroactive tempo-substituted acrylamide/lactide copolymer. Macromol Biosci. 2010;10:1203–9.
Oyaizu K, Ando Y, Konishi H, Nishide H. Nernstian adsorbate-like bulk layer of organic radical polymers for high-density charge storage purposes. J Am Chem Soc. 2008;130:14459–61.
Choi W, Ohtani S, Oyaizu K, Nishide H, Geckeler KE. Radical polymer-wrapped swnts at a molecular level: high-rate redox mediation through a percolation network for a transparent charge-storage material. Adv Mater. 2011;23:4440–3.
Gao H, Zhu Q, Neale AR, Bahri M, Wang X, Yang H, et al. Integrated covalent organic framework/carbon nanotube composite as Li-ion positive electrode with ultra-high rate performance. Adv Energy Mater. 2021;11:2101880.
Luo Z, Liu L, Ning J, Lei K, Lu Y, Li F, et al. A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew Chem Int Ed. 2018;57:9443–6.
Wang A, Tan R, Breakwell C, Wei X, Fan Z, Ye C, et al. Solution-processable redox-active polymers of intrinsic microporosity for electrochemical energy storage. J Am Chem Soc. 2022;144:17198–208.
Ma T, Li CH, Thakur RM, Tabor DP, Lutkenhaus JL. The role of the electrolyte in non-conjugated radical polymers for metal-free aqueous energy storage electrodes. Nat Mater. 2023;22:495–502.
Wang X, Dong H, Lakraychi AE, Zhang Y, Yang X, Zheng H, et al. Electrochemical swelling induced high material utilization of porous polymers in magnesium electrolytes. Mater Today. 2022;55:29–36.
Armand M, Tarascon JM. Building better batteries. Nature. 2008;451:652–57.
Oka K, Furukawa S, Murao S, Oka T, Nishide H, Oyaizu K. Poly(dihydroxybenzoquinone): its high-density and robust charge storage capability in rechargeable acidic polymer-air batteries. Chem Commun. 2020;56:4055–58.
Choi W, Harada D, Oyaizu K, Nishide H. Aqueous electrochemistry of poly(vinylanthraquinone) for anode-active materials in high-density and rechargeable polymer/air batteries. J Am Chem Soc. 2011;133:19839–43.
Kawai T, Oyaizu K, Nishide H. High-density and robust charge storage with poly(anthraquinone-substituted norbornene) for organic electrode-active materials in polymer-air secondary batteries. Macromolecules. 2015;48:2429–34.
Oyaizu K, Choi W, Nishide H. Functionalization of poly(4-chloromethylstyrene) with anthraquinone pendants for organic anode-active materials. Polym Adv Technol. 2011;22:1242–47.
Sasada Y, Langford SJ, Oyaizu K, Nishide H. Poly(norbornyl-NDIs) as a potential cathode-active material in rechargeable charge storage devices. RSC Adv. 2016;6:42911–16.
Oyaizu K, Hatemata A, Choi W, Nishide H. Redox-active polyimide/carbon nanocomposite electrodes for reversible charge storage at negative potentials: expanding the functional horizon of polyimides. J Mater Chem. 2010;20:5404–10.
Kato F, Kikuchi A, Okuyama T, Oyaizu K, Nishide H. Nitroxide radical molecules as highly reactive redox mediators in dye-sensitized solar cells. Angew Chem Int Ed. 2012;124:10324–27.
Kato F, Hayashi N, Murakami T, Okumura C, Oyaizu K, Nishide H. Nitroxide radicals for highly efficient redox mediation in dye-sensitized solar cells. Chem Lett. 2010;39:464–5.
Oyama N, Tatsuma T, Sato T, Sotomura T. Dimercaptan-polyaniline composite electrodes for lithium batteries with high energy density. Nature. 1995;373:598–600.
Madec L, Bouvrée A, Blanchard P, Cougnon C, Brousse T, Lestriez B, et al. In situ redox functionalization of composite electrodes for high power-high energy electrochemical storage systems via a non-covalent approach. Energy Environ Sci. 2012;5:5379–86.
Iwakura C, Kawai T, Nojima M, Yoneyama H. A new electrode‐active material for polymer batteries: polyvinylferrocene. J Electrochem Soc. 1987;134:791–4.
Yonekuta Y, Oyaizu K, Nishide H. Structural implication of oxoammonium cations for reversible organic one-electron redox reaction to nitroxide radicals. Chem Lett. 2007;36:866–7.
Yonekuta Y, Susuki K, Oyaizu K, Honda K, Nishide H. Battery-inspired non-volatile and rewritable memory architectures: a radical polymer-based organic device. J Am Chem Soc. 2007;129:14128–9.
Oyaizu K, Kawamoto T, Suga T, Nishide H. Synthesis and charge transport properties of redox-active nitroxide polyethers with large site density. Macromolecules. 2010;43:10382–9.
Suga T, Takeuchi S, Ozaki T, Sakata M, Oyaizu K, Nishide H. Synthesis of poly(oxoammonium salt)s and their electrical properties in the organic thin film device. Chem Lett. 2009;38:1160–61.
Oyaizu K, Suga T, Yoshimura K, Nishide H. Synthesis and characterization of radical-bearing polyethers as an electrode-active material for organic secondary batteries. Macromolecules. 2008;41:6646–52.
Takahashi Y, Hayashi N, Oyaizu K, Honda K, Nishide H. Totally organic polymer-based electrochromic cell using tempo-substituted polynorbornene as a counter electrode-active material. Polym J. 2008;40:763–7.
Kato R, Kato F, Oyaizu K, Nishide H. Redox-active hydroxy-TEMPO radical immobilized in Nafion layer for an aqueous electrolyte-based and dye-sensitized solar cell. Chem Lett. 2014;43:480–2.
Oyaizu K, Sukegawa T, Nishide H. Dual dopable poly(phenylacetylene) with nitronyl nitroxide pendants for reversible ambipolar charging and discharging. Chem Lett. 2011;40:184–5.
Suga T, Sugita S, Ohshiro H, Oyaizu K, Nishide H. P- and N-Type bipolar redox-active radical polymer: toward totally organic polymer-based rechargeable devices with variable configuration. Adv Mater. 2011;23:751–4.
Sukegawa T, Kai A, Oyaizu K, Nishide H. Synthesis of pendant nitronyl nitroxide radical-containing poly(norbornene)s as ambipolar electrode-active materials. Macromolecules. 2013;46:1361–7.
Suga T, Ohshiro H, Sugita S, Oyaizu K, Nishide H. Emerging n-type redox active radical polymer for a totally organic polymer-based rechargeable battery. Adv Mater. 2009;21:1627–30.
Xiang J, Sato K, Tokue H, Oyaizu K, Ho CL, Nishide H, et al. Synthesis and charge-discharge properties of organometallic copolymers of ferrocene and triphenylamine as cathode active materials for organic-battery applications. Eur J Inorg Chem. 2016;2016:1030–5.
Meng Z, Sato K, Sukegawa T, Oyaizu K, Ho CL, Xiang J, et al. Metallopolyyne polymers with ferrocenyl pendant ligands as cathode-active materials for organic battery application. J Organomet Chem. 2016;812:51–5.
Maruo H, Tanaka S, Takamura M, Oyaizu K, Segawa H, Nishide H. Oxoammonium cation of TEMPO: a very efficient dopant for hole-transporting triaryl amines in a perovskite solar cell. MRS Commun. 2018;8:122–6.
Wylie L, Kempt R, Heine T, Oyaizu K, Karton A, Yoshizawa-Fujita M, et al. Toward improved performance of all-organic nitroxide radical batteries with ionic liquids: a theoretical perspective. ACS Sustain Chem Eng. 2019;7:5367–75.
Wylie L, Blesch T, Freeman R, Hatakeyama-Sato K, Oyaizu K, Yoshizawa-Fujita M, et al. Reversible reduction of the TEMPO radical: one step closer to an all-organic redox flow battery. ACS Sustain Chem Eng. 2020;8:17988–96.
Wylie L, Hatakeyama-Sato K, Go C, Oyaizu K, Izgorodina E. Electrochemical characterization and thermodynamic analysis of TEMPO derivatives in ionic liquids. Phys Chem Chem Phys. 2021;23:10205–17.
Zhang K, Xie Y, Noble BB, Monteiro MJ, Lutkenhaus JL, Oyaizu K, et al. Unraveling kinetics and mass transport effects on two-electron storage in radical polymer batteries. J Mater Chem A. 2021;9:13071–9.
Maruo H, Oyaizu K, Nishide H. Electrochemical formation of a polyviologen-ZNO composite with an efficient charging capability. Chem Lett. 2015;44:393–5.
Suzuka M, Hara S, Sekiguchi T, Oyaizu K, Nishide H. Polyviologen as the charge-storage electrode of an aqueous electrolyte- and organic-based dye-sensitized solar cell. Polymer. 2015;68:353–7.
Hatakeyama-Sato K, Ichinoi R, Sasada Y, Sasaki Y, Oyaizu K, Nishide H. n-Type redox-active benzoylpyridinium-substituted supramolecular gel for an organogel-based rechargeable device. Chem Lett. 2019;48:555–7.
Armand M, Grugeon S, Vesin H, Laruelle S, Ribière P, Poizot P, et al. Conjugated dicarboxylate anodes for Li-ion batteries. Nat Mater. 2009;8:120–5.
Han X, Qing G, Sun J, Sun T. How many lithium ions can be inserted onto fused C6 aromatic ring systems? Angew Chem Int Ed. 2012;51:5147–51.
Renault S, Oltean VA, Araujo CM, Grigoriev A, Edström K, Brandell D. Superlithiation of organic electrode materials: the case of dilithium benzenedipropiolate. Chem Mater. 2006;28:1920–6.
Yang H, Liu S, Cao L, Jiang S, Hou H. Superlithiation of non-conductive polyimide toward high-performance lithium-ion batteries. J Mater Chem A. 2018;6:21216–24.
Nakahara K, Oyaizu K, Nishide H. Electrolyte anion-assisted charge transportation in poly(oxoammonium cation/nitroxyl radical) redox gels. J Mater Chem. 2012;22:13669–73.
Sasada Y, Ichinoi R, Oyaizu K, Nishide H. Supramolecular organic radical gels formed with 2,2,6,6-tetramethylpiperidin-1-oxyl-substituted cyclohexanediamines: a very efficient charge-transporting and -storable soft material. Chem Mater. 2017;29:5942–7.
Hatakeyama-Sato K, Wakamatsu H, Matsumoto S, Sadakuni K, Matsuoka K, Nagatsuka T, et al. TEMPO-substituted poly(ethylene sulfide) for solid-state electrochemical charge storage. Macromol Rapid Commun. 2021;42:2000607.
Hyakutake T, Park JY, Yonekuta Y, Oyaizu K, Nishide H, Advincula R. Nanolithographic patterning via electrochemical oxidation of stable poly(nitroxide radical)s to poly(oxoammonium salt)s. J Mater Chem. 2010;20:9616–8.
Sukegawa T, Omata H, Masuko I, Oyaizu K, Nishide H. Anionic polymerization of 4-methacryloyloxy-TEMPO using an MMA-capped initiator. ACS Macro Lett. 2014;3:240–3.
Sato K, Sukegawa T, Oyaizu K, Nishide H. Synthesis of poly(TEMPO-substituted glycidyl ether) by utilizing t-BuOK/18-crown-6 for an organic cathode-active material. Macromol Symp. 2015;351:90–6.
Yoshihara S, Isozumi H, Kasai M, Yonehara H, Ando Y, Oyaizu K, et al. Improving charge/discharge properties of radical polymer electrodes influenced strongly by current collector/carbon fiber interface. J Phys Chem B. 2010;114:8335–40.
Yoshihara S, Katsuta H, Isozumi H, Kasai M, Oyaizu K, Nishide H. Designing current collector/composite electrode interfacial structure of organic radical battery. J Power Sour. 2011;196:7806–11.
Choi W, Endo S, Oyaizu K, Nishide H, Geckeler KE. Robust and efficient charge storage by uniform grafting of TEMPO radical polymer around multi-walled carbon nanotubes. J Mater Chem A. 2013;1:2999–3003.
Sukegawa T, Sato K, Oyaizu K, Nishide H. Efficient charge transport of a radical polyether/SWCNT composite electrode for an organic radical battery with high charge-storage density. RSC Adv. 2015;5:15448–52.
Sato K, Wakamatsu H, Katagiri R, Oyaizu K, Nishide H. An ultrahigh output rechargeable electrode of a hydrophilic radical polymer/nanocarbon hybrid with an exceptionally large current density beyond 1 A cm−2. Adv Mater. 2018;30:1800900.
Hatakeyama-Sato K, Mizukami R, Serikawa T, Oyaizu K, Nishide H. A highly flexible yet >300 mAh cm-3 energy density lithium-ion battery assembled with the cathode of a redox-active polyether binder. Energy Technol. 2020;8:1901159.
Oyaizu K, Tatsuhira H, Nishide H. Facile charge transport and storage by a TEMPO-populated redox mediating polymer integrated with polyaniline as electrical conducting path. Polym J. 2015;47:212–9.
Oka K, Strietzel C, Emanuelsson R, Nishide H, Oyaizu K, Strømme M, et al. Characterization of PEDOT-quinone conducting redox polymers in water-in-salt electrolytes for safe and high-energy Li-ion batteries. Electrochem Commun. 2019;105:106489.
Oka K, Strietzel C, Emanuelsson R, Nishide H, Oyaizu K, Strømme M, et al. Conducting redox polymer as a robust organic electrode-active material in acidic aqueous electrolyte towards polymer-air secondary batteries. ChemSusChem. 2020;13:2280–5.
Oka K, Löfgren R, Emanuelsson R, Nishide H, Oyaizu K, Strømme M, et al. Conducting redox polymer as organic anode material for polymer-manganese secondary batteries. ChemElectroChem. 2020;7:3336–40.
Liang Y, Jing Y, Gheytani S, Lee KY, Liu P, Facchetti A, et al. Universal quinone electrodes for long cycle life aqueous rechargeable batteries. Nat Mater. 2017;16:841–8.
Molina A, Patil N, Ventosa E, Liras M, Palma J, Marcilla R. Electrode engineering of redox-active conjugated microporous polymers for ultra-high areal capacity organic batteries. ACS Energy Lett. 2020;5:2945–53.
Russell JC, Posey VA, Gray J, May R, Reed DA, Zhang H, et al. High-performance organic pseudocapacitors via molecular contortion. Nat Mater. 2021;20:1136–41.
Speer ME, Sterzenbach C, Esser B. Evaluation of cyclooctatetraene-based aliphatic polymers as battery materials: synthesis, electrochemical, and thermal characterization supported by DFT calculations. ChemPlusChem. 2017;82:1274–81.
Kolek M, Otteny F, Schmidt P, Mück-Lichtenfeld C, Einholz C, Becking J, et al. Ultra-high cycling stability of poly(vinylphenothiazine) as a battery cathode material resulting from π–π interactions. Energy Environ Sci. 2017;10:2334–41.
Lee B, Kang K. Long-lived electrodes for plastic batteries. Nature. 2017;549:339–40.
Otteny F, Kolek M, Becking J, Winter M, Bieker P, Esser B. Unlocking full discharge capacities of poly(vinylphenothiazine) as battery cathode material by decreasing polymer mobility through cross-linking. Adv Energ Mater. 2018;8:1802151.
Speer ME, Kolek M, Jassoy JJ, Heine J, Winter M, Bieker PM, et al. Thianthrene-functionalized polynorbornenes as high-voltage materials for organic cathode-based dual-ion batteries. Chem Commun. 2015;51:15261–4.
Gomez I, Leonet O, Blazquez JA, Grande HJ, Mecerreyes D. Poly(anthraquinonyl sulfides): high capacity redox polymers for energy storage. ACS Macro Lett. 2018;7:419–24.
Kawai T, Nakao S, Nishide H, Oyaizu K. Poly(diphenanthrenequinone-substituted norbornene) for long life and efficient lithium battery cathodes. Bull Chem Soc Jpn. 2018;91:721–7.
Nokami T, Matsuo T, Inatomi Y, Hojo N, Tsukagoshi T, Yoshizawa H, et al. Polymer-bound pyrene-4,5,9,10-tetraone for fast-charge and -discharge lithium-ion batteries with high capacity. J Am Chem Soc. 2012;134:19694–700.
Ueberricke L, Mildner F, Wu Y, Thauer E, Wickenhäuser T, Zhang WS, et al. Redox-active, porous pyrene tetraone dendritic polymers as cathode materials for lithium-ion batteries. Mater Adv. 2023;4:1604–11.
Takahashi T, Korolev K, Tsuji K, Oyaizu K, Nishide H, Bryuzgin E, et al. Facile grafting-onto-preparation of block copolymers of TEMPO and glycidyl methacrylates on an oxide substrate as an electrode-active layer. Polymer. 2015;68:310–14.
Zhang K, Hu Y, Wang L, Monteiro MJ, Jia Z. Pyrene-functionalized PTMA by NRC for greater π–π stacking with rGO and enhanced electrochemical properties. ACS Appl Mater Interfaces. 2017;9:34900–08.
Tokue H, Murata T, Agatsuma H, Nishide H, Oyaizu K. Charge-discharge with rocking-chair-type Li+ migration characteristics in a zwitterionic radical copolymer composed of TEMPO and trifluoromethanesulfonylimide with carbonate electrolytes for a high-rate Li-ion battery. Macromolecules. 2017;50:1950–8.
Chae IS, Koyano M, Oyaizu K, Nishide H. Self-doping inspired zwitterionic pendant design of radical polymers toward a rocking-chair-type organic cathode-active material. J Mater Chem A. 2013;1:1326–33.
Chae IS, Koyano M, Sukegawa T, Oyaizu K, Nishide H. Redox equilibrium of a zwitterionic radical polymer in a non-aqueous electrolyte for novel Li+ host material in a Li-ion battery. J Mater Chem A. 2013;1:9608–11.
Hatakeyama-Sato K, Matsumoto S, Takami H, Nagatsuka T, Oyaizu K. A PROXYL-type norbornene polymer for high-voltage cathodes in lithium batteries. Macromol Rapid Commun. 2021;42:2100374.
Hatakeyama-Sato K, Go C, Akahane T, Kaseyama T, Yoshimoto T, Oyaizu K. Quadruply fused aromatic heterocycles toward 4 V-class robust organic cathode-active materials. Batteries Supercaps. 2022;5:e202200178.
Hatakeyama-Sato K, Matsumoto S, Aida I, Oyaizu K. Anomalous potential shifts of redox-active molecules in highly concentrated electrolytes. Chem Lett. 2021;50:1375–7.
Oka K, Kato R, Oyaizu K, Nishide H. Poly(vinyldibenzothiophenesulfone): its redox capability at very negative potential toward an all-organic rechargeable device with high-energy density. Adv Funct Mater. 2018;28:1805858.
Perticarari S, Grange E, Doizy T, Quarez E, Oyaizu K, Guyomard D, et al. Full organic aqueous battery based on TEMPO small molecule with millimeter-thick electrodes. Chem Mater. 2019;31:1869–80.
Oka K, Murao S, Kobayashi K, Nishide H, Oyaizu K. Charge- and proton-storage capability of naphthoquinone-substituted poly(allylamine) as electrode-active material for polymer-air secondary batteries. ACS Appl Energy Mater. 2020;3:12019–24.
Oka K, Murao S, Kakaoka M, Nishide H, Oyaizu K. Hydrophilic anthraquinone-substituted polymer: its environmentally friendly preparation and efficient charge/proton-storage capability for polymer-air secondary batteries. Macromolecules. 2021;54:4854–9.
Hatakeyama-Sato K, Tezuka T, Ichinoi R, Matsumono S, Sadakuni K, Oyaizu K. Metal-free, solid-state, and paper-like rechargeable batteries consisting of redox-active polyethers. ChemSusChem. 2020;13:2443–8.
Hatakeyama-Sato K, Wakamatsu H, Yamagishi K, Fujie T, Takeoka S, Oyaizu K, et al. Ultrathin and stretchable rechargeable devices with organic polymer nanosheets conformable to skin surface. Small. 2019;15:1805296.
Ho JC, Hatakeyama-Sato K, Chiba A, Hayashi M, Igarashi Y, Oyaizu K, et al. Sandwich configuration of zinc anode, gel electrolyte, and radical polymer cathode for fully stretch-rechargeable battery. Adv Sustain Syst. 2023;7:2300080.
Hatakeyama-Sato K, Igarashi Y, Oyaizu K. Charge-transport kinetics of dissolved redox-active polymers for rational design of flow batteries. RSC Adv. 2023;13:547–57.
Ding C, Zhang H, Li X, Liu T, Xing F. Vanadium flow battery for energy storage: prospects and challenges. J Phys Chem Lett. 2013;4:1281–94.
Deller Z, Jones LA, Maniam S. Aqueous redox flow batteries: how ‘green’ are the redox active materials? Green Chem. 2021;23:4955–79.
Noack J, Roznyatovskaya N, Herr T, Fischer P. The chemistry of redox-flow batteries. Angew Chem Int Ed. 2015;54:9776–809.
Soloveichik GL. Flow batteries: current status and trends. Chem Rev. 2015;115:11533–58.
Fang X, Li Z, Zhao Y, Yue D, Zhang L, Wei X. Multielectron organic redoxmers for energy-dense redox flow batteries. ACS Mater Lett. 2022;4:277–306.
Lai YY, Li X, Zhu Y. Polymeric active materials for redox flow battery application. ACS Appl Polym Mater. 2020;2:113–28.
Burgess M, Moore JS, Rodríguez-López J. Redox active polymers as soluble nanomaterials for energy storage. Acc Chem Res. 2016;49:2649–57.
Machado CA, Brown GO, Yang R, Hopkins TE, Pribyl JG, Epps TH III. Redox flow battery membranes: improving battery performance by leveraging structure-property relationships. ACS Energy Lett. 2021;6:158–76.
Winsberg J, Hagemann T, Janoschka T, Hager MD, Schubert US. Redox-flow batteries: from metals to organic redox-active materials. Angew Chem Int Ed. 2017;56:686–711.
Hatakeyama-Sato K, Nagano T, Noguchi S, Sugai Y, Du J, Nishide H, et al. Hydrophilic organic redox-active polymer nanoparticles for higher energy density flow batteries. ACS Appl Polym Mater. 2019;1:188–96.
Sukegawa T, Masuko I, Oyaizu K, Nishide H. Expanding the dimensionality of polymers populated with organic robust radicals toward flow cell application: synthesis of TEMPO-crowded bottlebrush polymers using anionic polymerization and ROMP. Macromolecules. 2014;47:8611–17.
Oyama N, Ohsaka T. Voltammetric diagnosis of charge transport on polymer coated electrodes, Murray RW. Molecular design of electrode surfaces. New York: John Wiley & Sons; 1992.
Tokue H, Oyaizu K, Sukegawa T, Nishide H. TEMPO/viologen electrochemical heterojunction for diffusion controlled redox mediation: a highly rectifying bilayer-sandwiched device based on cross reaction at interface between dissimilar redox polymers. ACS Appl Mater Interfaces. 2014;6:4043–49.
Tokue H, Kakitani K, Nishide H, Oyaizu K. Electrochemical current rectification with cross reaction at a TEMPO/viologen-substituted polymer thin-layer heterojunction. RSC Adv. 2016;6:99195–201.
Tokue H, Kakitani K, Nishide H, Oyaizu K. Redox mediation through TEMPO-substituted polymer with nanogap electrodes for electrochemical amplification. Chem Lett. 2017;46:647–50.
Sato K, Yamasaki T, Mizuma T, Oyaizu K, Nishide H. Dynamic switching of ionic conductivity by cooperative interaction of polyviologen and liquid crystals for efficient charge storage. J Mater Chem A. 2016;4:3249–52.
Sato K, Yamasaki T, Nishide H, Oyaizu K. Grafted radical polymer brush for surface-driven switching of chiral nematic liquid crystals. Polym J. 2017;49:691–3.
Sato K, Mizuma T, Nishide H, Oyaizu K. Command surface of self-organizing structures by radical polymers with cooperative redox reactivity. J Am Chem Soc. 2017;139:13600–3.
Martin HJ, Hughes BK, Braunecker WA, Gennett T, Dadmun MD. The impact of radical loading and oxidation on the conformation of organic radical polymers by small angle neutron scattering. J Mater Chem A. 2018;6:15659–67.
Kemper TW, Gennett T, Larsen RE. Molecular dynamics simulation study of solvent and state of charge effects on solid-phase structure and counterion binding in a nitroxide radical containing polymer energy storage material. J Phys Chem C. 2016;120:25639–46.
Wang S, Li F, Easley AD, Lutkenhaus JL. Real-time insight into the doping mechanism of redox-active organic radical polymers. Nat Mater. 2019;18:69–75.
Sato K, Ichinoi R, Mizukami R, Serikawa T, Sasaki Y, Lutkenhaus J, et al. Diffusion-cooperative model for charge transport by redox-active nonconjugated polymers. J Am Chem Soc. 2018;140:1049–56.
Hatakeyama-Sato K, Masui T, Serikawa T, Sasaki Y, Choi W, Doo SG, et al. Non- conjugated redox-active polymer mediators for rapid electrocatalytic charging of lithium metal oxides. ACS Appl Energy Mater. 2019;2:6375–82.
Vlad A, Singh N, Rolland J, Melinte S, Ajayan PM, Gohy JF. Hybrid supercapacitor-battery materials for fast electrochemical charge storage. Sci Rep. 2014;4:4315.
Dolphijn G, Isikli S, Gauthy F, Vlad A, Gohy JF. Hybrid LiMn2O4-radical polymer cathodes for pulse power delivery applications. Electrochim Acta. 2017;255:442–8.
Suwa K, Oyaizu K, Segawa H, Nishide H. Anti-oxidizing radical polymer-incorporated perovskite layers and their photovoltaic characteristics in solar cells. ChemSusChem. 2019;12:5207–12.
Hatakeyama-Sato K, Akahane T, Go C, Kaseyama T, Yoshimoto T, Oyaizu K. Ultrafast charge/discharge by a 99.9% conventional lithium iron phosphate electrode containing 0.1% redox-active fluoflavin polymer. ACS Energy Lett. 2020;5:1712–7.
Hatakeyama-Sato K, Go C, Kaseyama T, Yoshimoto T, Oyaizu K. Accelerating charge/discharge of lithium iron phosphate by charge mediation reaction of poly(dimethylfluoflavin-substituted norbornene). Chem Lett. 2022;51:1040–3.
Hatakeyama-Sato K, Sadakuni K, Kitagawa K, Oyaizu K. Thianthrene polymers as 4 V-class organic mediators for redox targeting reaction with LiMn2O4 in flow batteries. Sci Rep. 2023;13:5711.
Yamamoto K, Suemasa D, Masuda K, Aita K, Endo T. Hyperbranched triphenylamine polymer for ultrafast battery cathode. ACS Appl Mater Interfaces. 2018;10:6346–53.
Bergner BJ, Schürmann A, Peppler K, Garsuch A, Janek J. TEMPO: a mobile catalyst for rechargeable Li-O2 batteries. J Am Chem Soc. 2014;136:15054–64.
Kojima Y. Hydrogen storage materials for hydrogen and energy carriers. Int J Hydrog Energy. 2019;44:18179–92.
Schlapbach L, Züttel A. Hydrogen-storage materials for mobile applications. Nature. 2001;414:353–8.
Gianotti E, Taillades-Jacquin M, Rozière J, Jones DJ. High-purity hydrogen generation via dehydrogenation of organic carriers: a review on the catalytic process. ACS Catal. 2018;8:4660–80.
Baum ZJ, Diaz LL, Konovalova T, Zhou QA. Materials research directions toward a green hydrogen economy: a review. ACS Omega. 2022;7:32908–35.
Kim TW, Jeong H, Baik JH, Suh YW. State-of-the-art catalysts for hydrogen storage in liquid organic hydrogen carriers. Chem Lett. 2022;51:239–55.
Kato R, Yoshimasa K, Egashira T, Oya T, Oyaizu K, Nishide H. A ketone/alcohol polymer for cycle of electrolytic hydrogen-fixing with water and -releasing under mild conditions. Nat Commun. 2016;7:13032.
Kato R, Oka K, Yoshimasa K, Nakajima M, Nishide H, Oyaizu K. Reversible hydrogen releasing and fixing with poly(vinylfluorenol) through a mild Ir-catalyzed dehydrogenation and electrochemical hydrogenation. Macromol. Rapid Commun. 2019;40:1900139.
Oka K, Kaiwa Y, Kataoka M, Fujita K, Oyaizu K. A polymer sheet-based hydrogen carrier. Eur J Org Chem. 2020;2020:5876–9.
Miyake J, Ogawa Y, Tanaka T, Ahn J, Oka K, Oyaizu K, et al. Rechargeable proton exchange membrane fuel cell containing an intrinsic hydrogen storage polymer. Commun Chem 2020;3:138.
Kato R, Oya T, Shimazaki Y, Oyaizu K, Nishide H. A hydrogen-storing quinaldine polymer: nickel-electrodeposition-assisted hydrogenation and subsequent hydrogen evolution. Polym Int. 2017;66:647–52.
Oka K, Kaiwa Y, Furukawa S, Nishide H, Oyaizu K. Reversible hydrogen fixation and release under mild conditions by poly(vinylquinoxaline). ACS Appl Polym Mater. 2020;2:2756–60.
Kaiwa Y, Oka K, Nishide H, Oyaizu K. Facile reversible hydrogenation of a poly(6-vinyl-2,3-dimethyl-1,2,3,4-tetrahydroquinoxaline) gel-like solid. Polym Adv Technol. 2021;32:1162–7.
Oka K, Kataoka M, Nishide H, Oyaizu K. Poly(vinyl diphenylquinoxaline) as a hydrogen storage material toward rapid hydrogen evolution. MRS Commun. 2022;12:213–6.
Oka K, Tobita Y, Kataoka M, Murao S, Kobayashi K, Furukawa S, et al. Synthesis of vinyl polymers substituted with 2-propanol and acetone and investigation of their reversible hydrogen storage capability. Polym J. 2021;53:799–804.
Oka K, Tobita Y, Kataoka M, Kobayashi K, Kaiwa Y, Nishide H, et al. Hydrophilic isopropanol/acetone-substituted polymers for safe hydrogen storage. Polym Int. 2022;71:348–51.
Oka K, Kataoka M, Kaiwa Y, Oyaizu K. Alcohol-substituted vinyl polymers for stockpiling hydrogen. Bull Chem Soc Jpn. 2021;94:2770–3.
Oka K, Kaiwa Y, Kobayashi K, Tobita Y, Oyaizu K. Accelerating the dehydrogenation reaction of alcohols by introducing them into poly(allylamine). Polym Chem. 2023;14:2588–91.
Fujita K, Tanaka Y, Kobayashi M, Yamaguchi R. Homogeneous perdehydrogenation and perhydrogenation of fused bicyclic N-heterocycles catalyzed by iridium complexes bearing a functional bipyridonate ligand. J Am Chem Soc. 2014;136:4829–32.
Kumar A, Janes T, Espinosa-Jalapa NA, Milstein D. Selective hydrogenation of cyclic imides to diols and amines and its application in the development of a liquid organic hydrogen carrier. J Am Chem Soc. 2018;140:7453–7.
Fujita K, Wada T, Shiraishi T. Reversible interconversion between 2,5-dimethylpyrazine and 2,5-dimethylpiperazine by iridium-catalyzed hydrogenation/dehydrogenation for efficient hydrogen storage. Angew Chem Int Ed. 2017;129:11026–9.
Mikami Y, Ebata K, Mitsudome T, Mizugaki T, Jitsukawa K, Kaneda K. Reversible dehydrogenation-hydrogenation of tetrahydroquinoline-quinoline using a supported copper nanoparticle catalyst. Heterocycles. 2010;82:1371–7.
Deraedt C, Ye R, Ralston WT, Toste FD, Somorjai GA. Dendrimer-stabilized metal nanoparticles as efficient catalysts for reversible dehydrogenation/hydrogenation of N-heterocycles. J Am Chem Soc. 2017;139:18084–92.
Modisha PM, Ouma CNM, Garidzirai R, Wasserscheid P, Bessarabov D. The prospect of hydrogen storage using liquid organic hydrogen carriers. Energy Fuels. 2019;33:2778–96.
Shimbayashi T, Fujita K. Metal-catalyzed hydrogenation and dehydrogenation reactions for efficient hydrogen storage. Tetrahedron. 2020;76:1–28.
Zhang L, Qiu R, Xue X, Pan Y, Xu C, Li H, et al. Versatile (pentamethylcyclopentadienyl)rhodium-2,2′-bipyridine (Cp*Rh-bpy) catalyst for transfer hydrogenation of N-heterocycles in water. Adv Synth Catal. 2015;357:3529–37.
Wang S, Huang H, Bruneau C, Fischmeister C. Iridium-catalyzed hydrogenation and dehydrogenation of N-heterocycles in water under mild conditions. ChemSusChem. 2019;12:2350–4.
Preuster P, Papp C, Wasserscheid P. Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy. Acc Chem Res. 2017;50:74–85.
Aakko-Saksa PT, Cook C, Kiviaho J, Repo T. Liquid organic hydrogen carriers for transportation and storing of renewable energy - review and discussion. J Power Sour. 2018;396:803–23.
Dean D, Davis B, Jessop PG. The effect of temperature, catalyst and sterics on the rate of N-heterocycle dehydrogenation for hydrogen storage. N. J Chem. 2011;35:417–22.
Crabtree RH. Hydrogen storage in liquid organic heterocycles. Energy Environ Sci. 2008;1:134–8.
Fujita K. Development and application of new iridium catalysts for efficient dehydrogenative reactions of organic molecules. Bull Chem Soc Jpn. 2019;92:344–51.
Kawahara R, Fujita K, Yamaguchi R. Cooperative catalysis by iridium complexes with a bipyridonate ligand: versatile dehydrogenative oxidation of alcohols and reversible dehydrogenation-hydrogenation between 2-propanol and acetone. Angew Chem Int Ed. 2012;51:12790–4.
Kawahara R, Fujita K, Yamaguchi R. Dehydrogenative oxidation of alcohols in aqueous media using water-soluble and reusable Cp*Ir catalysts bearing a functional bipyridine ligand. J Am Chem Soc. 2012;134:3643–6.
Moromi SK, Siddiki SMAH, Kon K, Toyao T, Shimizu K. Acceptorless dehydrogenation of N-heterocycles by supported Pt catalysts. Catal Today. 2017;281:507–11.
Eblagon KM, Rentsch D, Friedrichs O, Remhof A, Zuettel A, Ramirez-Cuesta AJ, et al. Hydrogenation of 9-ethylcarbazole as a prototype of a liquid hydrogen carrier. Int J Hydrog Energy. 2010;35:11609–21.
Eblagon KM, Tam K, Tsang SCE. Comparison of catalytic performance of supported ruthenium and rhodium for hydrogenation of 9-ethylcarbazole for hydrogen storage applications. Energy Environ Sci. 2012;5:8621–30.
Matsunaga T, Kubota T, Sugimoto T, Satoh M. High-performance lithium secondary batteries using cathode active materials of triquinoxalinylenes exhibiting six electron migration. Chem Lett. 2011;40:750–2.
Brushett FR, Vaughey JT, Jansen AN. An all-organic non-aqueous lithium-ion redox flow battery. Adv Energy Mater. 2012;2:1390–6.
Yan NF, Li GR, Gao XP. Electroactive organic compounds as anode-active materials for solar rechargeable redox flow battery in dual-phase electrolytes. J Electrochem Soc. 2014;161:A736–41.
Leung P, Aili D, Xu Q, Rodchanarowan A, Shah AA. Rechargeable organic-air redox flow batteries. Sustain Energy Fuels. 2018;2:2252–9.
Onoda M, Nagano Y, Fujita K. Iridium-catalyzed dehydrogenative lactonization of 1,4-butanediol and reversal hydrogenation: new hydrogen storage system using cheap organic resources. Int J Hydrog Energy. 2019;44:28514–20.
Acknowledgements
This work was partially supported by Grants-in-Aid for Scientific Research (Nos. 18H05515, 21H04695 and 22K18335) from MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oyaizu, K. Reversible and high-density energy storage with polymers populated with bistable redox sites. Polym J 56, 127–144 (2024). https://doi.org/10.1038/s41428-023-00857-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00857-7