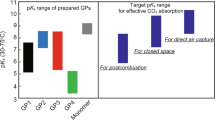
Abstract
Capturing CO2 from various sources, such as postcombustion exhaust gases and the atmosphere, is essential for a sustainable human society. Effective CO2 separation materials such as adsorbents and membranes are of utmost importance in efficient CO2 capture. This short review is focused on CO2 separation materials consisting of hydrogel particles. The first chapter introduces stimuli-responsive micro- and nanogel particles that reversibly absorb CO2 in response to thermal stimuli. The development of temperature-responsive hydrogel films comprising gel particles for reversible CO2 capture is introduced. The importance of choosing amines with optimal pKa values for efficient CO2 capture from various sources is explained in detail. The assembly of CO2 separation membranes consisting of amine-containing hydrogel particles is introduced in the final chapter. The paper highlights the promise of separation materials consisting of hydrogel particles for efficient CO2 capture from postcombustion gases and air and the prospects for further advances in this area.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goeppert A, Czaun M, Prakash GKS, Olah GA. Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere. Energy Environ Sci. 2012;5:7833.
Sanz-Perez ES, Murdock CR, Didas SA, Jones CW. Direct capture of CO2 from Ambient Air. Chem Rev. 2016;116:11840.
Bui M, Adjiman CS, Bardow A, Anthony EJ, Boston A, Fennell SBPS. et al. Carbon capture and storage (CCS): the way forward. Energy Environ Sci. 2018;11:1062
Choi S, Drese JH, Jones CW. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem. 2009;2:796–854.
Lee KB, Beaver MG, Caram HS, Sircar S. Reversible chemisorbents for carbon dioxide and their potential applications. Ind Eng Chem Res. 2008;47:8048–62.
Choi S, Gray ML, Jones CW. Amine-tethered solid adsorbents coupling high adsorption capacity and regenerability for CO2 capture from ambient air. ChemSusChem. 2011;4:628–35.
Harlick PJE, Sayari A. Applications of pore-expanded mesoporous silica. 5. Triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance. Ind Eng Chem Res. 2007;46:446–58.
Serna-Guerrero R, Da’na E, Sayari A. New insights into the interactions of CO2 with amine-functionalized silica. Ind Eng Chem Res. 2008;47:9406–12.
Goeppert A, Czaun M, May RB, Prakash GKS, Olah GA, Narayanan SR. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J Am Chem Soc. 2011;133:20164–7.
Park HB, Kamcev J, Robeson LM, Elimelech M, Freeman BD. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science (1979). 2017;356:eaab0530.
Merkel TC, Lin H, Wei X, Baker R. Power plant post-combustion carbon dioxide capture: an opportunity for membranes. J Membr Sci. 2010;359:126.
Du N, Park HB, Dal-Cin MM, Guiver MD. Advances in high permeability polymeric membrane materials for CO2 separation. Energy Environ Sci. 2012;5:7306.
Kim HW, Yoon HW, Yoon S-M, Yoo BM, Ahn BK, Cho YH, et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science (1979). 2013;342:91.
Peng Y, Li Y, Ban Y, Jin H, Jiao W, Liu X, et al. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science (1979). 2014;346:1356.
Jimenez-Solomon MF, Song Q, Jelfs KE, Munoz-Ibanez M, Livingston AG. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat Mater. 2016;15:760.
Chen Y, Ho WSW. High-molecular-weight polyvinylamine/piperazine glycinate membranes for CO2 capture from flue gas. J Membr Sci. 2016;514:376.
Qiao Z, Zhao S, Sheng M, Wang J, Wang S, Wang Z, et al. Metal-induced ordered microporous polymers for fabricating large-area gas separation membranes. Nat Mater. 2018;18:163.
Jeon MY, Kim D, Kumar P, Lee PS, Rangnekar N, Bai P, et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature. 2017;543:690.
Vendamme R, Onoue S-Y, Nakao A, Kunitake T. Robust free-standing nanomembranes of organic/inorganic interpenetrating networks. Nat Mater. 2006;5:494.
Fu Y, Jiang Y-B, Dunphy D, Xiong H, Coker E, Chou SS, et al. Ultra-thin enzymatic liquid membrane for CO2 separation and capture. Nat Commun. 2018;9:990.
Hoshino Y, Gyobu T, Imamura K, Hamasaki A, Honda R, Horii R, et al. Assembly of defect-free microgel nanomembranes for CO2 separation. ACS Appl Mater Interfaces. 2021;13:30030–8.
Sagle AC, Ju H, Freeman BD, Sharma MM. PEG-based hydrogel membrane coatings. Polym (Guildf). 2009;50:756.
Ju H, Sagle AC, Freeman BD, Mardel JI, Hill AJ. Characterization of sodium chloride and water transport in crosslinked poly(ethylene oxide) hydrogels. J Membr Sci. 2010;358:131.
Wu Y, Joseph S, Aluru NR. Effect of cross-linking on the diffusion of water, ions, and small molecules in hydrogels. J Phys Chem B. 2009;113:3512.
Liu Y, Yu S, Wu H, Li Y, Wang S, Tian Z, et al. High permeability hydrogel membranes of chitosan/poly ether-block-amide blends for CO2 separation. J Membr Sci. 2014;469:198.
Yue M, Hoshino Y, Miura Y. Design rationale of thermally responsive microgel particle films that reversibly absorb large amounts of CO2: fine tuning the pKa of ammonium ions in the particles. Chem Sci. 2015;6:6112.
Honda R, Hamasaki A, Miura Y, Hoshino Y. Thermoresponsive CO2 absorbent for various CO2 concentrations: tuning the pKa of ammonium ions for effective carbon capture. Polym J. 2021;53:157.
Zhang X, Chen L, Lim KH, Gonuguntla S, Lim KW, Pranantyo D, et al. The pathway to intelligence: using stimuli-responsive materials as building blocks for constructing smart and functional systems. Adv Mater. 2019;31:1804540.
Lynch I, de Gregorio P, Dawson KA. Simultaneous release of hydrophobic and cationic solutes from thin-film “plum-pudding” gels: a multifunctional platform for surface drug delivery? J Phys Chem B. 2005;109:6257–61.
Nagase K, Kobayashi J, Kikuchi A, Akiyama Y, Kanazawa H, Okano T. Preparation of thermoresponsive cationic copolymer brush surfaces and application of the surface to separation of biomolecules. Biomacromolecules. 2008;9:1340–7.
Hoshino Y, Kodama T, Okahata Y, Shea KJ. Peptide imprinted polymer nanoparticles: a plastic antibody. J Am Chem Soc. 2008;130:15242–3.
Hoare T, Pelton R. Impact of microgel morphology on functionalized microgel−drug interactions. Langmuir. 2008;24:1005–12.
Hoshino Y, Haberaecker WWIII, Kodama T, Zeng Z, Okahata Y, Shea KJ. Affinity purification of multifunctional polymer nanoparticles. J Am Chem Soc. 2010;132:13648–50.
Rao TP, Kala R, Daniel S. Metal ion-imprinted polymers—novel materials for selective recognition of inorganics. Anal Chim Acta. 2006;578:105–16.
Yoshimatsu K, Lesel BK, Yonamine Y, Beierle JM, Hoshino Y, Shea KJ. Temperature-responsive “catch and release” of proteins by using multifunctional polymer-based nanoparticles. Angew Chem Int Ed. 2012;51:2405–8.
Hoshino Y, Miyoshi T, Nakamoto M, Miura Y. Wide-range pKa tuning of proton imprinted nanoparticles for reversible protonation of target molecules via thermal stimuli. J Mater Chem B. 2017;5:9204–10.
Hoshino Y, Ohashi RC, Miura Y. Rational design of synthetic nanoparticles with a large reversible shift of acid dissociation constants: proton imprinting in stimuli responsive nanogel particles. Adv Mater. 2014;26:3718–23.
Honda R, Gyobu T, Shimahara H, Miura Y, Hoshino Y. Electrostatic interactions between acid-/base-containing polymer nanoparticles and proteins: impact of polymerization pH. ACS Appl Bio Mater. 2020;3:3827–34.
Paril A, Alb AM, Giz AT, Çatalgil-Giz H. Effect of medium pH on the reactivity ratios in acrylamide acrylic acid copolymerization. J Appl Polym Sci. 2007;103:968–74.
Serpe MJ, Yarmey KA, Nolan CM, Lyon LA. Doxorubicin uptake and release from microgel thin films. Biomacromolecules. 2005;6:408–13.
Koide H, Saito K, Yoshimatsu K, Chou B, Hoshino Y, Yonezawa S, et al. Cooling-induced, localized release of cytotoxic peptides from engineered polymer nanoparticles in living mice for cancer therapy. J Controll Release. 2023;355:745–59.
Hoshino Y, Jibiki T, Nakamoto M, Miura Y. Reversible pKa modulation of carboxylic acids in temperature-responsive nanoparticles through imprinted electrostatic interactions. ACS Appl Mater Interfaces. 2018;10:31096–105.
Guo B, Miura Y, Hoshino Y. Rational design of thermocells driven by the volume phase transition of hydrogel nanoparticles. ACS Appl Mater Interfaces. 2021;13:32184–92.
Guo B, Hoshino Y, Gao F, Hayashi K, Miura Y, Kimizuka N, et al. Thermocells driven by phase transition of hydrogel nanoparticles. J Am Chem Soc. 2020;142:17318–22.
Kaneko Y, Yoshida R, Sakai K, Sakurai Y, Okano T. Temperature-responsive shrinking kinetics of poly (N-isopropylacrylamide) copolymer gels with hydrophilic and hydrophobic comonomers. J Memb Sci. 1995;101:13–22.
Wang J, Gan D, Lyon LA, El-Sayed MA. Temperature-Jump investigations of the kinetics of hydrogel nanoparticle volume phase transitions. J Am Chem Soc. 2001;123:11284–9.
Hoshino Y, Imamura K, Yue M, Inoue G, Miura Y. Reversible absorption of CO2 triggered by phase transition of amine-containing micro- and nanogel particles. J Am Chem Soc. 2012;134:18177–80.
Yue M, Imai K, Miura Y, Hoshino Y. Design and preparation of thermo-responsive vinylamine-containing micro-gel particles for reversible absorption of carbon dioxide. Polym J. 2017;49:601–6.
Gao J, Liu Y, Terayama Y, Katafuchi K, Hoshino Y, Inoue G. Polyamine nanogel particles spray-coated on carbon paper for efficient CO2 capture in a milli-channel reactor. Chem Eng J. 2020;401:126059.
Yue M, Hoshino Y, Ohshiro Y, Imamura K, Miura Y. Temperature-responsive microgel films as reversible carbon dioxide absorbents in wet environment. Angew Chem Int Ed. 2014;53:2654–7.
Yue M, Imai K, Yamashita C, Miura Y, Hoshino Y. Effects of hydrophobic modifications and phase transitions of polyvinylamine hydrogel films on reversible CO2 capture behavior: comparison between copolymer films and blend films for temperature-responsive CO2 absorption. Macromol Chem Phys. 2017;218:1600570.
Olah GA, Goeppert A, Prakash GKS. Beyond Oil and Gas: The Methanol Economy. Angew Chem Int Ed. 2005;44:2636–39.
Olah GA, Goeppert A, Prakash GKS. Chemical recycling of carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J Org Chem. 2009;74:487–98.
Rochelle GT. Amine scrubbing for CO2 capture. Science (1979). 2009;325:1652–4.
Fout T, Murphy JT. DOE/NETL’s Carbon Capture R&D Program for Existing Coal-Fired Power Plants. 2009.
Aaron D, Tsouris C. Separation of CO2 from flue gas: a review. Sep Sci Technol. 2005;40:321–48.
Davis JD. Thermal degradation of aqueous amines used for carbon dioxide capture. University of Texas, 2009.
Huang HY, Yang RT, Chinn D, Munson CL. Amine-grafted MCM-48 and silica xerogel as superior sorbents for acidic gas removal from natural gas. Ind Eng Chem Res. 2003;42:2427–33.
Park Y, Shin D, Jang YN, Park A-HA. CO2 capture capacity and swelling measurements of liquid-like nanoparticle organic hybrid materials via attenuated total reflectance fourier transform infrared spectroscopy. J Chem Eng Data. 2012;57:40–5.
D’Alessandro DM, Smit B, Long JR. Carbon dioxide capture: prospects for new materials. Angew Chem Int Ed. 2010;49:6058–82.
Choi S, Drese JH, Eisenberger PM, Jones CW. Application of amine-tethered solid sorbents for direct CO2 capture from the ambient air. Environ Sci Technol. 2011;45:2420–7.
Carbon Dioxide Capture from Existing Coal Fired Power Stations. 2007.
Pedersen PL. Biochemistry, 2nd edit., edited by Donald Voet and Judith G. Voet. New York: Wiley, 1995, 1392 pages, $86.95. Proteins: Structure, Function, and Genetics 1995;23:613.
Kilmartin JV, Breen JJ, Roberts GCK, Ho C. Direct measurement of the pK values of an alkaline Bohr group in human hemoglobin. Proc Natl Acad Sci. 1973;70:1246–9.
Shinmori H, Takeuchi M, Shinkai S. Spectroscopic detection of diols and sugars by a colour change in boronic acid-appended spirobenzopyrans. J Chem Soc, Perkin Transactions 2. 1996;1. https://doi.org/10.1039/p29960000001.
Shinkai S, Ogawa T, Nakaji T, Kusano Y, Nanabe O. Photocontrolled extraction ability of azobenzene-bridged azacrown ether. Tetrahedron Lett. 1979;20:4569–72.
Lynch I, Dawson KA. Release of model compounds from “plum-pudding”-type gels composed of microgel particles randomly dispersed in a gel matrix. J Phys Chem B. 2004;108:10893–8.
Tanaka T, Wang C, Pande V, Grosberg AY, English A, Masamune S, et al. Polymer gels that can recognize and recover molecules. Faraday Discuss. 1995;101:201.
Kavanagh CA, Rochev YA, Gallagher WM, Dawson KA, Keenan AK. Local drug delivery in restenosis injury: thermoresponsive co-polymers as potential drug delivery systems. Pharm Ther. 2004;102:1–15.
Yonamine Y, Hoshino Y, Shea KJ. ELISA-mimic screen for synthetic polymer nanoparticles with high affinity to target proteins. Biomacromolecules. 2012;13:2952–7.
Lee S-H, Hoshino Y, Randall A, Zeng Z, Baldi P, Doong R, et al. Engineered synthetic polymer nanoparticles as IgG affinity ligands. J Am Chem Soc. 2012;134:15765–72.
Akiyama Y, Kikuchi A, Yamato M, Okano T. Ultrathin Poly(N -isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir. 2004;20:5506–11.
Wulff G. Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev. 2002;102:1–28.
Mosbach K, Ramström O. The emerging technique of molecular imprinting and its future impact on biotechnology. Nat Biotechnol. 1996;14:163–70.
Feil H, Bae YH, Feijen J, Kim SW. Mutual influence of pH and temperature on the swelling of ionizable and thermosensitive hydrogels. Macromolecules. 1992;25:5528–30.
Nayak S, Debord SB, Lyon LA. Investigations into the deswelling dynamics and thermodynamics of thermoresponsive microgel composite films. Langmuir. 2003;19:7374–9.
Lynch I, Dawson KA. Synthesis and characterization of an extremely versatile structural motif called the “Plum-Pudding” gel. J Phys Chem B. 2003;107:9629–37.
Werz PDL, Kainz J, Rieger B. Thermo- and pH-responsive nanogel particles bearing secondary amine functionalities for reversible carbon dioxide capture and release. Macromolecules. 2015;48:6433–9.
Kainz J, Werz PDL, Troll C, Rieger B. Temperature and CO2 responsive polyethylenimine for highly efficient carbon dioxide release. RSC Adv. 2015;5:9556–60.
Du N, Park HB, Dal-Cin MM, Guiver MD. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ Sci. 2012;5:7306–22.
Taniguchi I, Kinugasa K, Toyoda M, Minezaki K, Tanaka H, Mitsuhara K. Piperazine-immobilized polymeric membranes for CO2 capture: mechanism of preferential CO2 permeation. Polym J. 2021;53:129–36.
Acknowledgements
This research was supported by JST ALCA, Japan (Grant No. JPMJAL1403), the Japan Aerospace Exploration Agency (JAXA) open innovation hub center, the MEXT Program: Data Creation and Utilization-Type Material Research and Development Project Grant Number JPMXP1122714694, JST Grant Number JPMJPF2114, and JCCL, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoshino, Y., Aki, S. Hydrogel particles for CO2 capture. Polym J 56, 463–471 (2024). https://doi.org/10.1038/s41428-023-00850-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00850-0