Abstract
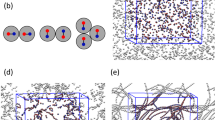
We determined the higher-order structures of polymer-brush-modified nanoparticles (PSiPs) in ionic liquids and explained the self-assembled structures as functions of the PSiP concentrations and brush lengths. The two types of brushes applied herein exhibited comparable structure formation patterns, suggesting that self-assembly of the PSiPs was entropy-driven. The crystallization threshold concentration of the PSiPs was understood through the Kirkwood–Alder transition in the assembly by considering the effective particle sizes. The crystal structure of the PSiP was characterized as a random hexagonal close-packed structure in the concentrated-polymer-brush regime, which exhibited the characteristics of hard spheres. In contrast, face-centered cubic (fcc) and body-centered cubic structures were observed in the semidilute-polymer-brush regime, reflecting softening of the interparticle potential. In addition, formation of the fcc structure was possibly due to partial compression and an imbalance in the swollen brush layer caused by the increased brush length and particle concentration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Holtz JH, Asher SA. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature. 1997;389:829–32. https://doi.org/10.1038/39834
Boden A, Bhave M, Wang PY, Jadhav S, Kingshott P. Binary Colloidal Crystal Layers as Platforms for Surface Patterning of Puroindoline-Based Antimicrobial Peptides. ACS Appl Mater Interfac. 2018;10:2264–74. https://doi.org/10.1021/acsami.7b10392
Yablonovitch E. Inhibited spontaneous emission in solid-state physics and electronics. Phys Rev Lett. 1987;58:2059–62. https://doi.org/10.1103/PhysRevLett.58.2059
John S. Strong localization of photons in certain disordered dielectric superlattices. Phys Rev Lett. 1987;58:2486–9. https://doi.org/10.1103/PhysRevLett.58.2486
Weissman JM, Sunkara HB, Tse AS, Asher SA. Thermally Switchable Periodicities and Diffraction from Mesoscopically Ordered Materials. Science. 1996;274:959–60. https://doi.org/10.1126/science.274.5289.959
Yang HS, Jang J, Lee BS, Kang TH, Park JJ, Yu WR. Redox-Triggered Coloration Mechanism of Electrically Tunable Colloidal Photonic Crystals. Langmuir. 2017;33:9057–65. https://doi.org/10.1021/acs.langmuir.7b01919
Lawrence JR, Ying Y, Jiang P, Foulger SH. Dynamic Tuning of Organic Lasers with Colloidal Crystals. Adv Mater. 2006;18:300–3. https://doi.org/10.1002/adma.200501833
Palberg T. Crystallization kinetics of repulsive colloidal spheres. J Phys: Condens Matter. 1999;11:R323–R360. https://doi.org/10.1088/0953-8984/11/28/201
Palberg T. Crystallization kinetics of colloidal model suspensions: recent achievements and new perspectives. J Phys: Condens Matter. 2014;26:333101 https://doi.org/10.1088/0953-8984/26/33/333101
Pusey PN, van Megen W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature. 1986;320:340–2. https://doi.org/10.1038/320340a0
Kegel WK, van Blaaderen A. Direct observation of dynamical heterogeneities in colloidal hard-sphere suspensions. Science. 2000;287:290–3. https://doi.org/10.1126/science.287.5451.290
Vanmegen W, Underwood SM. Change in crystallization mechanism at the glass transition of colloidal spheres. Nature. 1993;362:616–8. https://doi.org/10.1038/362616a0
Kose A, Hachisu S. Kirkwood–Alder transition in monodisperse latexes. I. Nonaqueous systems. J Coll Interfac Sci. 1974;46:460–9. https://doi.org/10.1016/0021-9797(74)90056-3
Cheng ZD, Russell WB, Chaikin PM. Controlled growth of hard-sphere colloidal crystals. Nature. 1999;401:893–5. https://doi.org/10.1038/44785
Zhu JX, Li M, Rogers R, Meyer W, Ottewill RH, Russell WB, et al. Crystallization of hard-sphere colloids in microgravity. Nature. 1997;387:883–5. https://doi.org/10.1038/43141
Hachisu S, Kobayashi Y, Kose A. Phase separation in monodisperse latexes. J Colloid Interfac Sci. 1973;42:342–8. https://doi.org/10.1016/0021-9797(73)90298-1
Hachisu S, Takano K. Pressure of disorder to order transition in monodisperse latex. Adv Colloid Interfac Sci. 1982;16:233–52. https://doi.org/10.1016/0001-8686(82)85018-5
Sogami IS, Yoshiyama T. Kossel line analysis on crystallization in colloidal suspensions. Phase Transitions. 1990;21:171–82. https://doi.org/10.1080/01411599008206889
Williams R, Crandall RS. The structure of crystallized suspensions of polystyrene spheres. Phys Lett A. 1974;48:225–6. https://doi.org/10.1016/0375-9601(74)90555-6
Clark NA, Hurd AJ, Ackerson BJ. Single colloidal crystals. Nature. 1979;281:57–60. https://doi.org/10.1038/281057a0
Yoshida H, Yamanaka J, Koga T, Ise N, Hashimoto T. Novel crystallization process in dilute ionic colloids. Langmuir. 1998;14:569–74. https://doi.org/10.1021/la970766l
Yoshida H, Ito K, Ise N. Localized ordered structure in polymer latex suspensions as studied by a confocal laser scanning microscope. Phys Rev B. 1991;44:435–8. https://doi.org/10.1103/PhysRevB.44.435
Hiltner PA, Krieger IM. Diffraction of light by ordered suspensions. J Phys Chem. 1969;73:2386–9. https://doi.org/10.1021/j100727a049
Okubo T. Polymer colloidal crystals. Prog Polym Sci. 1993;18:481–517. https://doi.org/10.1016/0079-6700(93)90015-5
Sirota EB, Ou-Yang HD, Sinha SK, Chaikin PM, Axe JD, Fujii Y. Complete phase diagram of a charged colloidal system: A synchro- tron x-ray scattering study. Phys Rev Lett. 1989;62:1524–7. https://doi.org/10.1103/PhysRevLett.62.1524
Monovoukas Y, Gast AP. The experimental phase diagram of charged colloidal suspensions. J Coll Interfac Sci. 1989;128:533–48. https://doi.org/10.1016/0021-9797(89)90368-8
Tsujii Y, Ohno K, Yamamoto S, Goto A, Fukuda T. Structure and Properties of High- Density Polymer Brushes Prepared by Surface-Initiated Living Radical Polymerization. Adv Polym Sci. 2006;197:1–45. https://doi.org/10.1007/12_063
Kobayashi M, Terayama Y, Kikuchi M, Takahara A. Chain dimensions and surface characterization of superhydrophilic polymer brushes with zwitterion side groups. Soft Matter. 2013;9:5138–48. https://doi.org/10.1039/c3sm27700c
Barbey R, Lavanant L, Paripovic D, Schuwer N, Sugnaux C, Tugulu S, et al. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chem Rev. 2009;109:5437–527. https://doi.org/10.1021/cr900045a
Ejaz M, Yamamoto S, Ohno K, Tsujii Y, Fukuda T. Controlled Graft Polymerization of Methyl Methacrylate on Silicon Substrate by the Combined Use of the Langmuir−Blodgett and Atom Transfer Radical Polymerization Techniques. Macromolecules. 1998;31:5934–6. https://doi.org/10.1021/ma980240n
Yamamoto S, Ejaz M, Tsujii Y, Matsumoto M, Fukuda T. Surface Interaction Forces of Well-Defined, High-Density Polymer Brushes Studied by Atomic Force Microscopy. 1. Effect of Chain Length. Macromolecules. 2000;33:5602–7. https://doi.org/10.1021/ma991733a
Yamamoto S, Ejaz M, Tsujii Y, Fukuda T. Surface interaction forces of well-defined, high-density polymer brushes studied by atomic force microscopy. 2 Effect of graft density. Macromolecules. 2000;33:5608–12. https://doi.org/10.1021/ma991988o
Nomura A, Ohno K, Fukuda T, Sato T, Tsujii Y. Lubrication mechanism of concentrated polymer brushes in solvents: effect of solvent viscosity. Polym Chem. 2012;3:148–53. https://doi.org/10.1039/c1py00215e
Yoshikawa C, Goto A, Tsujii Y, Fukuda T, Kimura T, Yamamoto K, et al. Protein Repellency of Well-Defined, Concentrated Poly(2-hydroxyethyl methacrylate) Brushes by the Size-Exclusion Effect. Macromolecules. 2006;39:2284–90. https://doi.org/10.1021/ma0520242
Yoshikawa C, Goto A, Tsujii Y, Ishizuka N, Nakanishi K, Fukuda T. Surface interaction of well-defined, concentrated poly(2-hydroxyethyl methacrylate) brushes with proteins. J Polym Sci, Part A: Polym Chem. 2007;45:4795–803. https://doi.org/10.1002/pola.22224
Ohno K, Morinaga T, Takeno S, Tsujii Y, Fukuda T. Suspensions of Silica Particles Grafted with Concentrated Polymer Brush: A New Family of Colloidal Crystals. Macromolecules. 2006;39:1245–9. https://doi.org/10.1021/ma0521708
Ohno K, Morinaga T, Takeno S, Tsujii Y, Fukuda T. Suspensions of Silica Particles Grafted with Concentrated Polymer Brush: Effects of Graft Chain Length on Brush Layer Thickness and Colloidal Crystallization. Macromolecules. 2007;40:9143–50. https://doi.org/10.1021/ma071770z
Morinaga T, Ohno K, Tsujii Y, Fukuda T. Structural Analysis of “Semisoft” Colloidal Crystals by Confocal Laser Scanning Microscopy. Macromolecules. 2008;41:3620–6. https://doi.org/10.1021/ma7028665
Morinaga T, Ohno K, Tsujii Y, Fukuda T. Two-dimensional ordered arrays of monodisperse silica particles grafted with concentrated polymer brushes. Eur Polym J. 2007;43:243–8. https://doi.org/10.1016/j.eurpolymj.2006.02.012
Huang Y, Morinaga T, Tai Y, Tsujii Y, Ohno K. Immobilization of semisoft colloidal crystals formed by polymer-brush-afforded hybrid particles. Langmuir. 2014;30:7304–12. https://doi.org/10.1021/la5011488
Huang Y, Takata A, Tsujii Y, Ohno K. Semisoft Colloidal Crystals in Ionic Liquids. Langmuir. 2017;33:7130–6. https://doi.org/10.1021/acs.langmuir.7b01449
Nakanishi Y, Ishige R, Ogawa H, Sakakibara K, Ohno K, Morinaga T, et al. USAXS analysis of concentration-dependent self-assembling of polymer- brush-modified nanoparticles in ionic liquid: [I] concentrated-brush regime. J Chem Phys. 2018;148:124902 https://doi.org/10.1063/1.5017552
Percus JK, Yevick GJ. Analysis of Classical Statistical Mechanics by Means of Collective Coordinates. Phys Rev. 1958;110:1–13. https://doi.org/10.1103/PhysRev.110.1
Wertheim MS. Exact Solution of the Percus–Yevick Integral Equation for Hard Spheres. Phys Rev Lett. 1963;10:321–3. https://doi.org/10.1103/PhysRevLett.10.321
Lebowitz JL. Exact Solution of Generalized Percus–Yevick Equation for a Mixture of Hard Spheres. Phys Rev. 1964;133:A895–A899. https://doi.org/10.1103/PhysRev.133.A895
Daoud M, Cotton JP. Star shaped polymers: a model for the conformation and its concentration dependence. J Phys (Paris). 1982;43:531–8. https://doi.org/10.1051/jphys:01982004303053100
Zhulina EB, Birshtein TM, Borisov OV. Curved polymer and polyelectrolyte brushes beyond the Daoud-Cotton model. Eur Phys J E. 2006;20:243–56. https://doi.org/10.1140/epje/i2006-10013-5
Kalb J, Dukes D, Kumar SK, Hoy RS, Grest GS. End grafted polymernanoparticles in a polymeric matrix: Effect of coverage and curvature. Soft Matter. 2011;7:1418–25. https://doi.org/10.1039/c0sm00725k
McConnell GA, Gast AP. Melting of Ordered Arrays and Shape Transitions in Highly Concentrated Diblock Copolymer Solutions. Macromolecules. 1997;30:435–44. https://doi.org/10.1021/ma961241n
McConnell GA, Gast AP, Huang JS, Smith SD. Disorder-order transitions in soft sphere polymer micelles. Phys Rev Lett. 1993;71:2102–5. https://doi.org/10.1103/PhysRevLett.71.2102
Lodge TP, Pudil B, Hanley KJ. The Full Phase Behavior for Block Copolymers in Solvents of Varying Selectivity. Macromolecules. 2002;35:4707–17. https://doi.org/10.1021/ma0200975
Hamley IW, Daniel C, Mingvanish W, Mai SM, Booth C, Messe L, et al. From Hard Spheres to Soft Spheres: The Effect of Copolymer Composition on the Structure of Micellar Cubic Phases Formed by Diblock Copolymers in Aqueous Solution. Langmuir. 2000;16:2508–14. https://doi.org/10.1021/la991035j
Laurati M, Stellbrink J, Lund R, Willner L, Richter D, Zaccarelli E. Starlike micelles with starlike interactions: a quantitative evaluation of structure factors and phase diagram. Phys Rev Lett. 2005; 94. https://doi.org/10.1103/PhysRevLett.94.195504
Laurati M, Stellbrink J, Lund R, Willner L, Zaccarelli E, Richter D. Asymmetric poly(ethylene-alt-propylene)-poly(ethylene oxide) micelles: A system with starlike morphology and interactions. Phys Rev E. 2007;76. https://doi.org/10.1103/PhysRevE.76.041503
Pedersen JS. Analysis of small-angle scattering data from colloids and polymer solutions: modeling and least-squares fitting. Adv Colloid Interfac Sci. 1997;70:171–210. https://doi.org/10.1016/s0001-8686(97)00312-6
Hashimoto T. Principles and Applications of X-ray, Light and Neutron Scattering. Singapore: Springer Nature Singapore Pte Ltd.; 2022.
Guinier A. X-ray Diffraction In Crystals, Imperfect Crystals, and Amorphous Bodies. New York: Dover; 1994.
Loose W, Ackerson BJ. Model-calculations for the analysis of scattering data from layered structures. J Chem Phys. 1994;101:7211–20. https://doi.org/10.1063/1.468278
Forster S, Timmann A, Konrad M, Schellbach C, Meyer A, Funari SS, et al. Scattering curves of ordered mesoscopic materials. J Phys Chem B. 2005;109:1347–60. https://doi.org/10.1021/jp0467494
Hoover WG, Ree FH. Use of Computer Experiments to Locate the Melting Transition and Calculate the Entropy in the Solid Phase. J Chem Phys. 1967;47:4873–8. https://doi.org/10.1063/1.1701730
Alder BJ, Hoover WG, Young DA. Studies in Molecular Dynamics. V. High-Density Equation of State and Entropy for Hard Disks and Spheres. J Chem Phys. 1968;49:3688–96. https://doi.org/10.1063/1.1670653
Acknowledgements
This work was partly supported by CREST, the Japan Science and Technology Agency, and an ICR Kyoto University Grant for Young Scientists. Synchrotron USAXS experiments were performed on BL03XU (Proposal Nos. 2017A1845 and 2017A7213), BL19B2 (Proposal Nos. 2014B1648, 2015A1718, and 2017B1638), and BL40B2 (Proposal Nos. 2014B1469 and 2022A1461) at SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI). The authors thank Drs Taizo Kabe, Hiroyasu Masunaga, Masugu Sato, Takeshi Watanabe, Keiichi Osaka, and Noboru Ohta (JASRI/SPring-8) for their assistance with the BL03XU, BL19B2, and BL40B2 experiments. The authors acknowledge support from the Quantum Beam Analyses Alliance (QBAA).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakanishi, Y., Ishige, R., Ogawa, H. et al. Unified explanation for self-assembly of polymer-brush-modified nanoparticles in ionic liquids. Polym J 55, 1199–1209 (2023). https://doi.org/10.1038/s41428-023-00829-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00829-x