Abstract
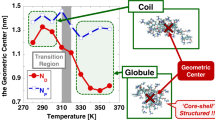
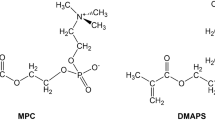
Cononsolvency refers to the phenomenon in which a polymer collapses in mixtures of good solvents with specific compositions. The coil-to-globule-to-coil transitions of a zwitterionic polymer, poly(2-[(N-2-methacryloyloxyethyl-N,N-dimethyl)ammonio]acetate) (PCB2), in water, ethanol, and water–ethanol mixed solvents were investigated and compared with those of poly[2-(methacryloyloxy)ethyl phosphorylcholine] (PMPC). PCB2 showed cononsolvency in water–ethanol mixed solvents with specific ethanol volume fractions. The reentrant square well-type sharp coil–globule transition was reproduced by a statistical mechanical model involving competitive hydrogen bonding and cooperative hydration. PCB2 showed a broader cononsolvency range than PMPC because of its lower association constants of water and ethanol and the marked competition for hydrogen bonding. The zwitterion-specific cononsolvency characteristics were rationalized with the electrostatic potentials and van der Waals energies of the zwitterions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kudaibergenov S, Jaeger W, Laschewsky A. Polymeric Betaines: Synthesis, Characterization, and Application. Adv Polym Sci. 2006;201:157–224.
Paschke S, Lienkamp K. Polyzwitterions: From Surface Properties and Bioactivity Profiles to Biomedical Applications. ACS Appl Polym Mater. 2020;2:129–51.
Nikkhah SJ, Vandichel M. Modeling Polyzwitterion-Based Drug Delivery Platforms: A Perspective of the Current State-of-the-Art and Beyond. ACS Eng Au. 2022;2:274–94.
Qian H, Wang K, Lv M, Zhao C, Wang H, Wen S, et al. Recent advances on next generation of polyzwitterion-based nano-vectors for targeted drug delivery. J Controlled Release. 2022;343:492–505.
Kobayashi M, Terayama Y, Yamaguchi H, Terada M, Murakami D, Ishihara K, et al. Wettability and Antifouling Behavior on the Surfaces of Superhydrophilic Polymer Brushes. Langmuir 2012;28:7212–22.
Kobayashi M, Terayama Y, Kikuchi M, Takahara A. Chain Dimensions and Surface Characterization of Superhydrophilic Polymer Brushes with Zwitterion Side Groups. Soft Matter. 2013;9:5138–48.
Jiang S, Cao Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv Mater. 2010;22:920–32.
Higaki Y, Kobayashi M, Murakami D, Takahara A. Anti-fouling Behavior of Polymer Brush Immobilized Surfaces. Polym J. 2016;48:325–31.
Chen M, Briscoe WH, Armes SP, Klein J. Lubrication at Physiological Pressures by Polyzwitterionic Brushes. Science. 2009;323:1698–701.
Kobayashi M, Terayama Y, Hosaka N, Kaido M, Suzuki A, Yamada N, et al. Friction Behavior of High-Density Poly(2-methacryloyloxyethyl phosphorylcholine) Brush in Aqueous Media. Soft Matter. 2007;3:740–6.
Ishihara K. Blood-Compatible Surfaces with Phosphorylcholine-Based Polymers for Cardiovascular Medical Devices. Langmuir 2019;35:1778–87.
Georgiev GS, Kamenska EB, Vassileva ED, Kamenova IP, Georgieva VT, Iliev SB, et al. Self-assembly, antipolyelectrolyte effect, and nonbiofouling properties of polyzwitterions. Biomacromolecules. 2006;7:1329–34.
Wang F, Yang J, Zhao J. Understanding anti-polyelectrolyte behavior of a well-defined polyzwitterion at the single-chain level. Polym Int. 2015;64:27045–51.
Laschewsky A, Rosenhahn A. Molecular Design of Zwitterionic Polymer Interfaces: Searching for the Difference. Langmuir. 2019;35:1056–71.
Hildebrand V, Laschewsky A, Päch M, Müller-Buschbaum P, Papadakis CM. Effect of the zwitterion structure on the thermo-responsive behaviour of poly(sulfobetaine methacrylates). Polym Chem. 2017;8:310–22.
Chevalier Y, Le Perchec P. Intercharge distance of flexible zwitterionic molecules in solution. J Phys Chem. 1990;94:1768–74.
Schlenoff JB. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir. 2014;30:9625–36.
Shimizu A, Hifumi E, Kojio K, Takahara A, Higaki Y. Modulation of Double Zwitterionic Block Copolymer Aggregates by Zwitterion-Specific Interactions. Langmuir. 2021;37:14760–6.
Higaki Y, Inutsuka Y, Sakamaki T, Terayama Y, Takenaka A, Higaki K, et al. Effect of Charged Group Spacer Length on Hydration State in Zwitterionic Poly(sulfobetaine) Brushes. Langmuir. 2017;33:8404–12.
Bharadwaj S, Niebuur BJ, Nothdurft K, Richtering W, van der Vegt NFA, Papadakis CM. Cononsolvency of thermoresponsive polymers: where we are now and where we are going. Soft Matter. 2022;18:2884–909.
Hirokawa Y, Tanaka T. Volume Phase Transition in a Nonionic Gel. J Chem Phys. 1984;81:6379–80.
Amiya T, Hirokawa Y, Hirose Y, Li Y, Tanaka T. Reentrant Phase Transition of N-isopropylacrylamide Gels in Mixed Solvents. J Chem Phys. 1987;86:2375.
Winnik FM, Ringsdorf H, Venzmer J. Methanol-Water as a Co-nonsolvent System for Poly(N-isopropylacrylamide). Macromolecules. 1990;23:2415–6.
Schild HG, Muthukumar M, Tirrell DA. Cononsolvency in Mixed Aqueous Solutions of Poly(N-isopropylacrylamide). Macromolecules. 1991;24:948–52.
Kreuzer LP, Lindenmeir C, Geiger C, Widmann T, Hildebrand V, Laschewsky A, et al. Poly(sulfobetaine) versus Poly(N‑isopropylmethacrylamide): Co‑Nonsolvency-Type Behavior of Thin Films in a Water/Methanol Atmosphere. Macromolecules. 2021;54:1548–56.
Zhang G, Wu C. The Water/Methanol Complexation Induced Reentrant Coil-to-Globule-to-Coil Transition of Individual Homopolymer Chains in Extremely Dilute Solution. J Am Chem Soc. 2001;123:1376–80.
Tanaka F, Koga T, Winnik FM. Temperature-Responsive Polymers in Mixed Solvents: Competitive Hydrogen Bonds Cause Cononsolvency. Phys Rev Lett. 2008;101:028302.
Mukherji D, Marques CM, Kremer K. Polymer collapse in miscible good solvents is a generic phenomenon driven by preferential adsorption. Nat Commun. 2014;5:4882.
Picaa A, Graziano G. An alternative explanation of the cononsolvency of poly(N-isopropylacrylamide) in water–methanol solutions. Phys Chem Chem Phys. 2016;18:25601–8.
Bharadwaj S, Nayar D, Dalgicdir C, van der Vegt NFA. An Interplay of Excluded-Volume and Polymer–(co)solvent Attractive Interactions Regulates Polymer Collapse in Mixed Solvents. J Chem Phys. 2021;154:134903.
Matsuda Y, Kobayashi M, Annaka M, Ishihara K, Takahara A. UCST-Type Cononsolvency Behavior of Poly(2-methacryloxyethyl phosphorylcholine) in the Mixture of Water and Ethanol. Polym J. 2008;40:479–83.
Edmondson S, Nguyen NT, Lewis AL, Armes SP. Co-Nonsolvency Effects for Surface-Initiated Poly(2-(methacryloyloxy)ethyl phosphorylcholine) Brushes in Alcohol/Water Mixtures. Langmuir. 2010;26:7216–26.
Ihara D, Higaki Y, Yamada NL, Nemoto F, Matsuda Y, Kojio K, et al. Cononsolvency of Poly[2-(methacryloyloxy)ethyl phosphorylcholine] in Ethanol−Water Mixtures: A Neutron Reflectivity Study.Langmuir. 2022;38:5081–8.
Takahashi M, Shimizu A, Yusa S-I, Higaki Y. Lyotropic Morphology Transition of Double Zwitterionic Diblock Copolymer Aqueous Solutions. Macromol Chem Phys. 2021;222:2000377.
Pérez-Ramírez HA, Haro-Pérez C, Odriozola G. Effect of Temperature on the Cononsolvency of Poly(N-isopropylacrylamide) (PNIPAM) in Aqueous 1-Propanol. ACS Appl Polym Mater. 2019;1:2961–72.
Otake K, Inomata H, Konno M, Saito S. Thermal Analysis of the Volume Phase Transition with N-isopropylacrylamide Gels. Macromolecules. 1990;23:283–9.
Ohta H, Ando I, Fujishige S, Kubota K. Molecular Motion and 1H NMR Relaxation of Aqueous Poly(N-isopropylacrylamide) Solution Under High Pressure. J Polym Sci B Polym Phys. 1991;29:963–8.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP22H02147 (Grant-in-Aid for Scientific Research (B)) and JP22H04555, JP19H05714 (Grant-in-Aid for Scientific Research on Innovative Area: Aquatic Functional Materials). Y.H. acknowledges the Tokuyama Science Foundation and the KOSÉ Cosmetology Research Foundation for financial support. This work was supported by the Oita University President’s Strategic Discretionary Fund. This work was partially supported by the Cooperative Research Program of the Network Joint Research Center for Materials and Devices. The authors acknowledge Y. Saruwatari (Osaka Organic Chemical Industry Ltd.) for a kind donation of the carboxybetaine methacrylate monomer. We thank D. Ihara for assistance with the statistical mechanical model simulations. We thank Kathryn Sole, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this paper.
Author information
Authors and Affiliations
Contributions
The paper was written with contributions from all authors, and all authors have approved the final version of the paper. Y.H. conceived and directed the project. N.K., T.M., and M.N. performed the experiments and analyzed the results. E.H. contributed to the DLS experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Higaki, Y., Kuraoka, N., Masuda, T. et al. Cononsolvency of poly(carboxybetaine methacrylate) in water–ethanol mixed solvents. Polym J 55, 869–876 (2023). https://doi.org/10.1038/s41428-023-00780-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00780-x