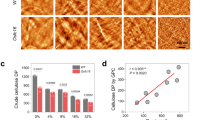
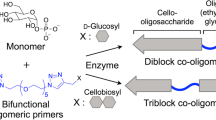
Abstract
The self-assembly of biomolecules is an important strategy for fabricating structurally ordered artificial nanomaterials. Various biopolymers have been utilized as self-assembling material components. However, crystalline polysaccharides, such as cellulose and chitin, have rarely been focused on as components for self-assembly, possibly due to their complicated chemical synthesis and low solubility in various solvents even though their stable and robust characteristics are observed in nature. Therefore, the development of methods to control the self-assembled structures of cellulose has the potential to extend the applicability of crystalline polysaccharides in materials science and engineering. In this study, we investigated the cellodextrin phosphorylase-catalyzed synthesis and self-assembled structures of cellulose oligomers in the presence of protein denaturants. The modulation of intermolecular interactions between oligomers by protein denaturants under adequate synthesis conditions resulted in the production of oligomers with greater degrees of polymerization and different crystal structures. Our findings will be significant as fundamental knowledge to artificially construct cellulose assemblies toward novel cellulosic materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Stupp SI, LeBonheur V, Walker K, Li LS, Huggins KE, Keser M, et al. Supramolecular materials: self-organized nanostructures. Science. 1997;276:384–9.
Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–21.
Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335:813–7.
Mattia E, Otto S. Supramolecular systems chemistry. Nat Nanotechnol. 2015;10:111–9.
Amabilino DB, Smith DK, Steed JW. Supramolecular materials. Chem Soc Rev. 2017;46:2404–20.
Komiyama M, Yoshimoto K, Sisido M, Ariga K. Chemistry can make strict and fuzzy controls for bio-systems: DNA nanoarchitectonics and cell-macromolecular nanoarchitectonics. Bull Chem Soc Jpn. 2017;90:967–1004.
Seeman NC, Sleiman HF. DNA nanotechnology. Nat Rev Mater. 2018;3:17068.
Hamada S, Luo D. Enzyme-based fabrication of physical DNA hydrogels: new materials and applications. Polym J. 2020;52:891–8.
Gatto E, Venanzi M. Self-assembled monolayers formed by helical peptide building blocks: a new tool for bioinspired nanotechnology. Polym J. 2013;45:468–80.
De Santis E, Ryadnov MG. Peptide self-assembly for nanomaterials: the old new kid on the block. Chem Soc Rev. 2015;44:8288–300.
Abbas M, Zou QL, Li SK, Yan XH. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv Mater. 2017;29:1605021.
Tsutsumi H, Matsubara D, Mihara H. Functionalization of self-assembling peptide materials using molecular recognition of supramolecular peptide nanofibers. Polym J. 2020;52:913–22.
Arakawa H, Takeda K, Higashi SL, Shibata A, Kitamura Y, Ikeda M. Self-assembly and hydrogel formation ability of Fmoc-dipeptides comprising α-methyl-L-phenylalanine. Polym J. 2020;52:923–30.
Luo Q, Hou CX, Bai YS, Wang RB, Liu JQ. Protein assembly: versatile approaches to construct highly ordered nanostructures. Chem Rev. 2016;116:13571–632.
Sawada T, Serizawa T. Filamentous viruses as building blocks for hierarchical self-assembly toward functional soft materials. Bull Chem Soc Jpn. 2018;91:455–66.
Numata K. How to define and study structural proteins as biopolymer materials. Polym J. 2020;52:1043–56.
Shimizu T. Self-assembly of discrete organic nanotubes. Bull Chem Soc Jpn. 2018;91:623–68.
Barriga HMG, Holme MN, Stevens MM. Cubosomes: the next generation of smart lipid nanoparticles? Angew Chem Int Ed. 2019;58:2958–78.
Sasaki Y, Akiyoshi K. Design and function of smart biomembrane nanohybrids for biomedical applications: review. Polym J. 2021;53:587–92.
Ifuku S, Saimoto H. Chitin nanofibers: preparations, modifications, and applications. Nanoscale. 2012;4:3308–18.
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40:3941–94.
Yataka Y, Suzuki A, Iijima K, Hashizume M. Enhancement of the mechanical properties of polysaccharide composite films utilizing cellulose nanofibers. Polym J. 2020;52:645–53.
Kobayashi S, Kashiwa K, Kawasaki T, Shoda S. Novel method for polysaccharide synthesis using an enzyme: the first in vitro synthesis of cellulose via a nonbiosynthetic path utilizing cellulase as catalyst. J Am Chem Soc. 1991;113:3079–84.
Lee JH, Brown RM, Kuga S, Shoda S, Kobayashi S. Assembly of synthetic cellulose I. Proc Natl Acad Sci USA. 1994;91:7425–9.
Egusa S, Kitaoka T, Goto M, Wariishi H. Synthesis of cellulose in vitro by using a cellulase/surfactant complex in a nonaqueous medium. Angew Chem Int Ed. 2007;46:2063–5.
Samain E, Lancelon-Pin C, Férigo F, Moreau V, Chanzy H, Heyraud A, et al. Phosphorolytic synthesis of cellodextrins. Carbohydr Res. 1995;271:217–26.
Hiraishi M, Igarashi K, Kimura S, Wada M, Kitaoka M, Samejima M. Synthesis of highly ordered cellulose II in vitro using cellodextrin phosphorylase. Carbohydr Res. 2009;344:2468–73.
Serizawa T, Kato M, Okura H, Sawada T, Wada M. Hydrolytic activities of artificial nanocellulose synthesized via phosphorylase-catalyzed enzymatic reactions. Polym J. 2016;48:539–44.
Serizawa T, Fukaya Y, Sawada T. Self-assembly of cellulose oligomers into nanoribbon network structures based on kinetic control of enzymatic oligomerization. Langmuir. 2017;33:13415–22.
Nakai H, Kitaoka M, Svensson B, Ohtsubo K. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr Opin Chem Biol. 2013;17:301–9.
O’Neill EC, Field RA. Enzymatic synthesis using glycoside phosphorylases. Carbohydr Res. 2015;403:23–37.
Yataka Y, Sawada T, Serizawa T. Enzymatic synthesis and post-functionalization of two-dimensional crystalline cellulose oligomers with surface-reactive groups. Chem Commun. 2015;51:12525–8.
Yataka Y, Sawada T, Serizawa T. Multidimensional self-assembled structures of alkylated cellulose oligomers synthesized via in vitro enzymatic reactions. Langmuir. 2016;32:10120–5.
Nohara T, Sawada T, Tanaka H, Serizawa T. Enzymatic synthesis of oligo(ethylene glycol)-bearing cellulose oligomers for in situ formation of hydrogels with crystalline nanoribbon network structures. Langmuir. 2016;32:12520–6.
Nohara T, Sawada T, Tanaka H, Serizawa T. Enzymatic synthesis and protein adsorption properties of crystalline nanoribbons composed of cellulose oligomer derivatives with primary amino groups. J Biomater Sci Polym Ed. 2017;28:925–38.
Wang J, Niu J, Sawada T, Shao Z, Serizawa T. A bottom-up synthesis of vinyl-cellulose nanosheets and their nanocomposite hydrogels with enhanced strength. Biomacromolecules. 2017;18:4196–205.
Sugiura K, Sawada T, Tanaka H, Serizawa T. Enzyme-catalyzed propagation of cello-oligosaccharide chains from bifunctional oligomeric primers for the preparation of block co-oligomers and their crystalline assemblies. Polym J. 2021;53:1133–43.
Hata Y, Fukaya Y, Sawada T, Nishiura M, Serizawa T. Biocatalytic oligomerization-induced self-assembly of crystalline cellulose oligomers into nanoribbon networks assisted by organic solvents. Beilstein J Nanotechnol. 2019;10:1778–88.
Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011;63:1118–59.
Li DF, Caffrey M. Renaturing membrane proteins in the lipid cubic phase, a nanoporous membrane mimetic. Sci Rep. 2014;4:5806.
Shiraki K, Tomita S, Inoue N. Small amine molecules: solvent design toward facile improvement of protein stability against aggregation and inactivation. Curr Pharm Biotechnol. 2016;17:116–25.
Mason PE, Brady JW, Neilson GW, Dempsey CE. The interaction of guanidinium ions with a model peptide. Biophys J. 2007;93:L4–L6.
O’Brien EP, Dima RI, Brooks B, Thirumalai D. Interactions between hydrophobic and ionic solutes in aqueous guanidinium chloride and urea solutions: lessons for protein denaturation mechanism. J Am Chem Soc. 2007;129:7346–53.
Kano F, Shinjo M, Qin ZJ, Li JS, Matsumura Y, Shimizu A, et al. Denaturant-induced helix-coil transition of oligopeptides: theoretical and equilibrium studies of short oligopeptides C17 and AK16. Polym J. 2011;43:293–300.
Ganguly P, Shea JE. Distinct and nonadditive effects of urea and guanidinium chloride on peptide solvation. J Phys Chem Lett. 2019;10:7406–13.
Mirdha L, Chakraborty H. Fluorescence quenching by ionic liquid as a potent tool to study protein unfolding intermediates. J Mol Liq. 2020;312:113408.
Zou Q, Habermann-Rottinghaus SM, Murphy KP. Urea effects on protein stability: hydrogen bonding and the hydrophobic effect. Proteins Struct Funct Bioinform. 1998;31:107–15.
Ganguly P, Polak J, van der Vegt NFA, Heyda J, Shea JE. Protein stability in TMAO and mixed urea-TMAO solutions. J Phys Chem B. 2020;124:6181–97.
Isogai A, Atalla RH. Dissolution of cellulose in aqueous NaOH solutions. Cellulose. 1998;5:309–19.
Petrovic DM, Kok I, Woortman AJJ, Ciric J, Loos K. Characterization of oligocellulose synthesized by reverse phosphorolysis using different cellodextrin phosphorylases. Anal Chem. 2015;87:9639–46.
Hishikawa Y, Togawa E, Kondo T. Characterization of individual hydrogen bonds in crystalline regenerated cellulose using resolved polarized FTIR spectra. ACS Omega. 2017;2:1469–76.
Isogai A, Usuda M, Kato T, Uryu T, Atalla RH. Solid-state CP/MAS 13C NMR study of cellulose polymorphs. Macromolecules. 1989;22:3168–72.
French AD. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose. 2014;21:885–96.
Buleon A, Chanzy H. Single crystals of cellulose IVII: preparation and properties. J Polym Sci B Polym Phys. 1980;18:1209–17.
Zugenmaier P. Conformation and packing of various crystalline cellulose fibers. Prog Polym Sci. 2001;26:1341–417.
Serizawa T, Maeda T, Sawada T. Neutralization-induced self-assembly of cellulose oligomers into antibiofouling crystalline nanoribbon networks in complex mixtures. ACS Macro Lett. 2020;9:301–5.
Serizawa T, Maeda T, Yamaguchi S, Sawada T. Aqueous suspensions of cellulose oligomer nanoribbons for growth and natural filtration-based separation of cancer spheroids. Langmuir 2020;36:13890–8.
Hata Y, Kojima T, Maeda T, Sawada T, Serizawa T. pH-Triggered self-assembly of cellulose oligomers with gelatin into a double-network hydrogel. Macromol Biosci. 2020;20:2000187.
Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282.
Tanford C. Protein denaturation: Part C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95.
Acknowledgements
The authors are grateful for the financial support to T. Serizawa from a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JP18H02029 and JP21H01996) and a Grant-in-Aid for Scientific Research on Innovative Areas (Aquatic Functional Materials) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (JP20H05208). The authors also gratefully acknowledge the technical support for the MALDI-TOF mass spectrometry and WAXD measurements provided by the Open Facility Center, Materials Analysis Division (Tokyo Tech).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sakurai, Y., Sawada, T. & Serizawa, T. Phosphorylase-catalyzed synthesis and self-assembled structures of cellulose oligomers in the presence of protein denaturants. Polym J 54, 561–569 (2022). https://doi.org/10.1038/s41428-021-00592-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00592-x
This article is cited by
-
Special issue: Fundamentals and applications of carbohydrate polymers
Polymer Journal (2022)