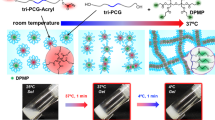
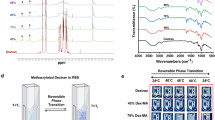
Abstract
Thermoresponsive and degradable hydrogels are considered promising materials for the development of smart drug delivery carriers that can be applied in the human body. However, the synthesis of a thermoresponsive degradable hydrogel with desirable properties is challenging. Here, we prepared thermoresponsive degradable copolymers and hydrogels by radical copolymerization of 2-methylene-1,3-dioxepane and N,N-dimethylacrylamide. The obtained polymers exhibited low critical solution temperature-type phase separation and a swelling-deswelling behavior. Under alkaline conditions (pH 11.3), these materials degraded and turned into water-soluble oligomers. In addition, the hydrogels self-degraded in PBS due to the decreased pH of the inner hydrogel. The prepared thermoresponsive degradable polymers and hydrogels have potential applicability as stimuli-responsive drug delivery carriers and cell culture scaffolds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Concheiro A, Alvarez-Lorenzo C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv Drug Deliv Rev 2013;65:1188–203.
Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2014;62:147–66.
Qui Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 2001;53:321–39.
Vileberghe SV, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387–408.
Billiet T, Vandenhaute M, Schelfhout J, Vlierberghe SV, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–41.
Schiphorst J, Coleman S, Stumpel JE, Azouz AB, Diamond D, Schenning APHJ. Molecular design of light-responsive hydrogels, for in situ generation of fast and reversible valves for microfluidic applications. Chem Mater 2015;27:5925–31.
Gan J, Guan X, Zheng J, Guo H, Wu K, Liang L, et al. Biodegradable, thermoresponsive PNIPAM-based hydrogel scaffolds for the sustained release of levofloxacin. RSC Adv. 2016;6:32967–78.
Zhao C, Zhuang X, He P, Xiao C, Sun J, Chen X, et al. Synthesis of biodegradable thermo- and pH-responsive hydrogels for controlled drug release. Polymer. 2009;50:4308–16.
Yamawaki K, Asoh T, Kikuchi A. Redox-responsive minimized fragmentation of three-armed oligo(ethylene glycol) gels for protein release. Colloid Surf B. 2016;146:343–51.
Aimetti A, Machen AJ, Anseth KS. Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials. 2009;30:6048–54.
Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam. Biomaterials. 2005;26:3055–64.
Aranaz I, Campos EM, Nash ME, Tardajos MG, Reinecke H, Elvira C, et al. Pseudo-double network hydrogels with unique properties as supports for cell manipulation. J Mater Chem B. 2014;2:3839–48.
Schmidt S, Zeiser M, Hellweg T, Duschl C, Fery A, Möhwald H. Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv Funct Mater 2010;20:3235–43.
Heskins M, Guillet JE. Solution properties of poly(N-isopropylacrylamide. J Macromol Sci. 1968;2:1441–55.
Chen Y, Wu H, Sun J, Dong G, Wang T. Injectable and thermoresponsive self-assembled nanocomposite hydrogel for long-term anticancer drug delivery. Langmuir. 2013;29:3721–9.
Galphrin A, Long TJ, Ratner BD. Degradable, thermo-sensitive poly(N-isopropyl acrylamide)-based scaffolds with controlled porosity for tissue engineering applications. Biomacromolecules. 2010;11:2583–92.
Komatsu S, Kayano H, Ando Y, Asoh T, Kikuchi A. Preparation of thermo- and redox-responsive branched polymers composed of three-armed oligo(ethylene glycol. J Polym Sci. 2018;56:2623–9.
Miyazaki H, Kataoka K. Preparation of polyacrylamide derivatives showing thermo-reversible coacervate formation and their potential application to two-phase separation processes. Polymer. 1996;37:681–5.
Maeda T, Takenouchi M, Yamamoto K, Aoyagi T. Analysis of the formation mechanism for thermoresponsive-type coacervate with Functional Copolymers Consisting ofN-Isopropylacrylamide and 2-Hydroxyisopropylacrylamide. Biomacromolecules. 2006;7:2230–6.
Yamamoto K, T Serizawa T, Akashi M. Synthesis and thermosensitive properties of Poly[(N‐vinylamide)‐co‐(vinyl acetate)]s and their hydrogels. Macromol Chem Phys. 2003;204:1027–33.
Komatsu S, Asoh T, Ishihara R, Kikuchi A. Facile preparation of degradable thermoresponsive polymers as biomaterials: Thermoresponsive polymers prepared by radical polymerization degrade to water-soluble oligomers. Polymer. 2017;130:68–73.
Komatsu S, Ikedo Y, Asoh T, Ishihara R, Kikuchi A. Fabrication of hybrid capsules via CaCO3 crystallization on degradable coacervate droplets. Langmuir. 2018;34:3981–6.
Komatsu S, Asoh T, Ishihara R, Kikuchi A. Fabrication of thermoresponsive degradable hydrogel made by radical polymerization of 2-methylene-1,3-dioxepane: Unique thermal coacervation in hydrogel. Polymer. 2019;179:121633.
Jackson AW. Reversible-deactivation radical polymerization of cyclic ketene acetals. Polym Chem 2020;11:3525–45.
Wairs U, Chennamaneni LR, Thoniyot P, Zhang H, Jackson AW. Main-chain degradable star polymers comprised of pH-responsive hyperbranched cores and thermoresponsive polyethylene glycol-based coronas. Polym Chem 2018;9:4824–39.
Yiu A, Simchuk D, Hao J. Facile synthesis of novel thermo‐responsive polyvalerolactones with tunable LCSTs. Macromol Chem Phys. 2020;221:2000136.
Bailey WJ, Ni Z, Wu SR. Synthesis of poly‐ϵ‐caprolactone via a free radical mechanism. Free radical ring‐opening polymerization of 2‐methylene‐1,3‐dioxepane. J Polym Chem Sci. 1982;20:3021–30.
Zhao, Pi B, Zhao L, Tian S, Ge J, Yang H, et al. RSC Adv., 2019;9:11833–41.
Tardy A, Nicolas J, Gigmes D, Lefay C, Guillaneuf Y. Radical ring-opening polymerization: scope, limitations, and application to (Bio)degradable materials. Chem Rev 2017;117:1319–406.
Hedir GG, Arno MC, Langlais M, Husband JT, O’Reilly RK, Dove AP. Poly(oligo(ethylene glycol) vinyl acetate)s: a versatile class of thermoresponsive and biocompatible polymers. Angew Chem Int Ed 2017;56:9178–82.
Undin J, Finne-Wistrand A, Albertsson A. Copolymerization of 2-methylene-1,3-dioxepane and glycidyl methacrylate, a well-defined and efficient process for achieving functionalized polyesters for covalent binding of bioactive molecules. Biomacromolecules. 2013;14:2095–102.
Asoh T, Nakajima T, Matsuyama T, Kikuchi A. Surface-functionalized biodegradable nanoparticles consisting of amphiphilic graft polymers prepared by radical copolymerization of 2-methylene-1,3-dioxepane and macromonomers. Langmuir. 2015;31:6879–85.
Lück M, Schröder W, Paulke BR, Blunk T, Müller RH. Complement activation by model drug carriers for intravenous application: determination by two-dimensional electrophoresis. Biomaterials. 1999;20:2063–8.
Ratchford N, Bangsaruntip S, Sun X, Welsher K, Dai H. Noncovalent functionalization of carbon nanotubes by fluorescein-polyethylene glycol: supramolecular conjugates with pH-dependent absorbance and fluorescence. J Am Chem Soc 2007;129:2448–9.
Low ZW, Chee PL, Kai D, Loh XJ. The role of hydrogen bonding in alginate/poly(acrylamide-co-dimethylacrylamide) and alginate/poly(ethylene glycol) methyl ether methacrylate-based tough hybrid hydrogels. RSC Adv. 2015;5:57678–85.
Caliceti P, Veronese FM. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)–protein conjugates. Adv Drug Deliv Rev 2003;55:1261–77.
Knauf J, Bell DP, Hirtzer P, Luo ZP, Young JD, Katre NV. Relationship of effective molecular size to systemic clearance in rats of recombinant interleukin-2 chemically modified with water-soluble polymers. J Biol Chem 1988;263:15064–70.
Huang MH, Li S, Vert M. Synthesis and degradation of PLA–PCL–PLA triblock copolymer prepared by successive polymerization of ε-caprolactone and dl-lactide. Polymer. 2004;45:8675–81.
Tran J, Pesenti T, Cressonnier J, Lefay C, Gigmes D, Guillaneuf Y, et al. Degradable copolymer nanoparticles from radical ring-opening copolymerization between cyclic ketene acetals and vinyl ethers. Biomacromolecules. 2019;20:305–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Komatsu, S., Sato, T. & Kikuchi, A. Facile preparation of 2-methylene-1,3-dioxepane-based thermoresponsive polymers and hydrogels. Polym J 53, 731–739 (2021). https://doi.org/10.1038/s41428-021-00463-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00463-5