Abstract
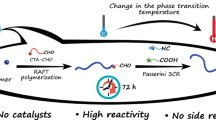
In this study, a novel pyrazole–carbodithioate-based chain transfer agent (CTA) featuring an aldehyde group (CTA-CHO) was designed and synthesized for RAFT polymerization. The obtained CTA-CHO was employed for the RAFT polymerization of styrene to afford well-defined polystyrenes bearing an aldehyde at their chain ends with low Ð values (~1.1). In addition, the reactivity of the aldehyde moiety at the end of the chain was precisely evaluated, while the Passerini three-component reaction was successfully performed on the aldehyde group.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Zhu J, Bienaymé H. Multicomponent reactions. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2005.
Armstrong RW, Combs AP, Tempest PA, Brown SD, Keating TA. Multiple-component condensation strategies for combinatorial library synthesis. Acc Chem Res. 1996;29:123–31. https://doi.org/10.1021/ar9502083
Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev. 2006;106:17–89. https://doi.org/10.1021/cr0505728
Dömling A, Wang W, Wang K. Chemistry and biology of multicomponent reactions. Chem Rev. 2012;112:3083–135. https://doi.org/10.1021/cr100233r
Rotstein BH, Zaretsky S, Rai V, Yudin AK. Small heterocycles in multicomponent reactions. Chem Rev. 2014;114:8323–59. https://doi.org/10.1021/cr400615v
Touré BB, Hall DG. Natural product synthesis using multicomponent reaction strategies. Chem Rev. 2009;109:4439–86. https://doi.org/10.1021/cr800296p
Wessjohann LA, Rivera DG, Vercillo OE. Multiple multicomponent macrocyclizations (MiBs): a strategic development toward macrocycle diversity. Chem Rev. 2009;109:796–814. https://doi.org/10.1021/cr8003407
Kreye O, Türünç O, Sehlinger A, Rackwitz J, Meier MAR. Structurally diverse polyamides obtained from monomers derived via the Ugi multicomponent reaction. Chem Eur J. 2012;18:5767–76. https://doi.org/10.1002/chem.201103341
Kreye O, Tóth T, Meier MAR. Introducing multicomponent reactions to polymer science: passerini reactions of renewable monomers. J Am Chem Soc. 2011;133:1790–2. https://doi.org/10.1021/ja1113003
Zhu C, Yang B, Zhao Y, Fu C, Tao L, Wei Y. A new insight into the Biginelli reaction: the dawn of multicomponent click chemistry? Polym Chem. 2013;4:5395–5400. https://doi.org/10.1039/C3PY00553D
Zhang Q, Zhang Y, Zhao Y, Yang B, Fu C, Wei Y, et al. Multicomponent Polymerization System Combining Hantzsch Reaction and Reversible Addition–Fragmentation Chain Transfer to Efficiently Synthesize Well-Defined Poly(1,4-dihydropyridine)s. ACS Macro Lett. 2015;4:128–32. https://doi.org/10.1021/mz500734c
Kakuchi R, Theato P. Three-Component Reactions for Post-Polymerization Modifications. ACS Macro Lett. 2013;2:419–22. https://doi.org/10.1021/mz400144q
Lee I-H, Kim H, Choi T-L. Cu-Catalyzed Multicomponent Polymerization To Synthesize a Library of Poly(N-sulfonylamidines). J Am Chem Soc. 2013;135:3760–3. https://doi.org/10.1021/ja312592e
Deng XX, Cui Y, Du FS, Li ZC. Functional highly branched polymers from multicomponent polymerization (MCP) based on the ABC type Passerini reaction. Polym Chem. 2014;5:3316–20. https://doi.org/10.1039/c3py01705b
Jee J-A, Spagnuolo LA, Rudick JG. Convergent synthesis of dendrimers via the passerini three-component reaction. Org Lett. 2012;14:3292–5. https://doi.org/10.1021/ol301263v
Li L, Kan X-W, Deng X-X, Song C-C, Du F-S, Li Z-C. Simultaneous dual end-functionalization of peg via the passerini three-component reaction for the synthesis of ABC miktoarm terpolymers. J Polym Sci Part A. 2013;51:865–73. https://doi.org/10.1002/pola.26443
Deng X-X, Li L, Li Z-L, Lv A, Du F-S, Li Z-C. Sequence Regulated Poly(ester-amide)s Based on Passerini Reaction. ACS Macro Lett. 2012;1:1300–3. https://doi.org/10.1021/mz300456p
Yang B, Zhao Y, Fu CK, Zhu CY, Zhang YL, Wang SQ, et al. Introducing the Ugi reaction into polymer chemistry as a green click reaction to prepare middle-functional block copolymers. Polym Chem. 2014;5:2704–8. https://doi.org/10.1039/c4py00001c
Kakuchi R, Theato P. Efficient multicomponent postpolymerization modification based on kabachnik-fields reaction. ACS Macro Lett. 2014;3:329–32. https://doi.org/10.1021/mz500139c
Moldenhauer F, Kakuchi R, Theato P. Synthesis of Polymers via Kabachnik-Fields Polycondensation. ACS Macro Lett. 2016;5:20–23. https://doi.org/10.1021/acsmacrolett.5b00720
Kakuchi R, Yoshida S, Sasaki T, Kanoh S, Maeda K. Multi-component post-polymerization modification reactions of polymers featuring lignin-model compounds. Polym Chem. 2018;9:2109–15. https://doi.org/10.1039/C7PY01923H
Zhang Y, Zhao Y, Yang B, Zhu C, Wei Y, Tao L. ‘One pot’ synthesis of well-defined poly(aminophosphonate)s: time for the Kabachnik–Fields reaction on the stage of polymer chemistry. Polym Chem. 2014;5:1857–62. https://doi.org/10.1039/C3PY01486J
Zhang Y, Zhao Y, Xia S, Tao L, Wei Y. A Facile Preparation of Mussel-Inspired Poly(dopamine phosphonate-co-PEGMA)s via a One-Pot Multicomponent Polymerization System. Macro Rapid Commun. 2020;41:1900533 https://doi.org/10.1002/marc.201900533
Iha RK, Wooley KL, Nyström AM, Burke DJ, Kade MJ, Hawker CJ. Applications of orthogonal click chemistries in the synthesis of functional soft materials. Chem Rev. 2009;109:5620–86. https://doi.org/10.1021/cr900138t
Golas PL, Matyjaszewski K. Marrying click chemistry with polymerization: expanding the scope of polymeric materials. Chem Soc Rev. 2010;39:1338–54. https://doi.org/10.1039/b901978m
Pound G, McKenzie JM, Lange RF, Klumperman B. Polymer-protein conjugates from omega-aldehyde endfunctional poly(N-vinylpyrrolidone) synthesised via xanthate-mediated living radical polymerisation. Chem Commun. 2008;27:3193–5. https://doi.org/10.1039/b803952f
Moad G, Rizzardo E, Thang SH. Living Radical Polymerization by the RAFT Process – A Third Update. Aust J Chem. 2012, 65 (8). https://doi.org/10.1071/ch12295.
Moad G. A Critical Survey of Dithiocarbamate Reversible Addition‐Fragmentation Chain Transfer (RAFT) Agents in Radical Polymerization. J Polym Sci Part A. 2018;57:216–27. https://doi.org/10.1002/pola.29199
Keddie DJ, Moad G, Rizzardo E, Thang SH. RAFT Agent Design and Synthesis. Macromolecules. 2012;45:5321–42. https://doi.org/10.1021/ma300410v
Li J, Yang S, Wang L, Wang X, Liu L. Thermoresponsive dynamic covalent polymers with tunable properties. Macromolecules. 2013;46:6832–42. https://doi.org/10.1021/ma400948j
Reader PW, Pfukwa R, Jokonya S, Arnott GE, Klumperman B. Synthesis of α,ω-heterotelechelic PVP for bioconjugation, via a one-pot orthogonal end-group modification procedure. Polym Chem. 2016;7:6450–6. https://doi.org/10.1039/C6PY01296E
Deng J, Liu X, Ma L, Cheng C, Sun S, Zhao C. Switching biological functionalities of biointerfaces via dynamic covalent bonds. J Mater Chem B. 2016;4:694–703. https://doi.org/10.1039/C5TB02072G
Jackson AW, Fulton DA. Dynamic Covalent Diblock Copolymers Prepared from RAFT Generated Aldehyde and Alkoxyamine End-Functionalized Polymers. Macromolecules. 2010;43:1069–75. https://doi.org/10.1021/ma902291a
Gardiner J, Martinez-Botella I, Tsanaktsidis J, Moad G. Dithiocarbamate RAFT agents with broad applicability – the 3,5-dimethyl-1H-pyrazole-1-carbodithioates. Polym Chem. 2016;7:481–92. https://doi.org/10.1039/c5py01382h
Gardiner J, Martinez-Botella I, Kohl TM, Krstina J, Moad G, Tyrell JH, et al. 4-Halogeno-3,5-dimethyl-1H-pyrazole-1-carbodithioates: versatile reversible addition fragmentation chain transfer agents with broad applicability. Polym Int. 2017;66:1438–47. https://doi.org/10.1002/pi.5423
Banerjee S, Guerre M, Améduri B, Ladmiral V. Syntheses of 2-(trifluoromethyl)acrylate-containing block copolymers via RAFT polymerization using a universal chain transfer agent. Polym Chem. 2018;9:3511–21. https://doi.org/10.1039/C8PY00655E
Willcock H, O’Reilly RK. End group removal and modification of RAFT polymers. Polym Chem. 2010;1:149–57. https://doi.org/10.1039/B9PY00340A
Uchiyama M, Satoh K, Kamigaito M. Cationic RAFT Polymerization Using ppm Concentrations of Organic Acid. Angew Chem Int Ed. 2015;54:1924–8. https://doi.org/10.1002/anie.201410858
Ramozzi R, Morokuma K. Revisiting the passerini reaction mechanism: existence of the nitrilium, organocatalysis of its formation, and solvent effect. J Org Chem. 2015;80:5652–7. https://doi.org/10.1021/acs.joc.5b00594
Maeda S, Komagawa S, Uchiyama M, Morokuma K. Finding reaction pathways for multicomponent reactions: the passerini reaction is a four-component reaction. Angew Chem Int Ed. 2011;50:644–9. https://doi.org/10.1002/anie.201005336
Sarri P, Venturi F, Cuda F, Roelens S. Binding of acetylcholine and tetramethylammonium to flexible cyclophane receptors: improving on binding ability by optimizing host’s geometry. J Org Chem. 2004;69:3654–61. https://doi.org/10.1021/jo049899j
Loim NM, Kelbyscheva ES. Synthesis of dendrimers with terminal formyl groups. Russ Chem Bull 2004;53:2080–5. https://doi.org/10.1007/s11172-005-0076-z
Acknowledgements
RK gratefully acknowledges the Leading Initiative for Excellent Young Researchers (LEADER) and a Grant-in-Aid for Scientific Research (C) (no. 19K05578) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kakuchi, R., Okura, Y. The Passerini three-component reaction of aldehyde end-functionalized polymers via RAFT polymerization using chain transfer agents featuring aldehyde. Polym J 52, 1057–1066 (2020). https://doi.org/10.1038/s41428-020-0368-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0368-z