Abstract
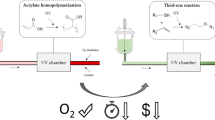
Radical photopolymerization of acrylate was carried out without a degassing process under conditions suitable for UV-curable coatings and UV-curable adhesives. We examined the differences in the resulting polymer structures obtained from the coating and adhesive by employing the same formulation. 2,2-Dimethoxy-2-phenylacetophenone was employed as a photoinitiator. The UV-curable coating yielded polymers terminated by either a hydroxyl group or a terminal carbonyl group as the main products. On the other hand, the UV-curable adhesive yielded a multitude of polymers that were terminated by disproportionation. In the latter case, some polymers underwent hydrogen abstraction from the polymer backbone, resulting in β-scission of the midchain radical to generate polymers with an α-substituted acryloyl group as the terminal group.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Hoyle CE. Photocurable coatings. In: Hoyle CE, Kinstle JF, editors. ACS Symposium Series 417: Radiation curing of polymeric materials. Washington, DC: American Chemical Society; 1990. p. 1–16.
Vanderhoff JW. Ultraviolet light cured inks-a review. In: Labana SS, editor. ACS Symposium Series: Ultraviolet light induced reactions in polymers. Washington, DC: American Chemical Society; 1976. p. 162–87.
Yagci Y, Jockusch S, Turro NJ. Photoinitiated polymerization: advance, challenges, and opportunities. Macromolecules. 2010;43:6245–60.
O’Brien AK, Bowman CN. Impact of oxygen on photopolymerization kinetics and polymer structure. Macromolecules. 2006;39:2501–6.
Krongauz VV, Chawla CP, Dupre J. Oxygen and radical photopolymerization in films. In: Belfield KD, Crivello JV, editors. ACS Symposium Series: Photoinitiated polymerization. Washington, DC: American Chemical Society; 2003; p. 165–75.
Decker C, Moussa K. UV-radiation- and laser-induced polymerization of acrylic monomers. In: Hoyle CE, Kinstle JF, editors. ACS Symposium Series 417: Radiation curing of polymeric materials. Washington, DC: American Chemical Society: 1990; p. 439–56.
Crivello JV, Reichmanis E. Photopolymer materials and processes for advanced technologies. Chem Mater. 2014;26:533–48.
Huber HF Radiation cure of pressure-sensitive adhesives. In: Fouassier JP, Rabek JF, editors. Radiation curing in polymer science and technology-volume IV practical aspects and applications. London and New York: Elsevier Applied Science; 1993. p. 51–72.
Ellerstein SM, Lee SA, Palit TK. Radiation-curable adhesives. In: Fouassier JP, Rabek JF, editors. Radiation curing in polymer science and technology-volume IV practical aspects and applications. London and New York: Elsevier Applied Science; 1993. p. 73–85.
Khudyakov IV, Legg JC, Purvis MB, Overton BJ. Kinetics of photopolymerization of acrylates with functionality of 1-6. Ind Eng Chem Res. 1999;38:3353–9.
Fouassier JP. An introduction to the basic principles in UV curing. In: Fouassier JP, Rabek JF, editors. Radiation curing in polymer science and technology-volume I fundamentals and methods. London and New York: Elsevier Applied Science; 1993. p. 49–113.
Kurdikar DL, Peppas NA. Method of determination of initiator efficiency: application to UV polymerizations using 2,2-dimethoxy-2-phenylacetophenone. Macromolecules. 1994;27:733–8.
Szablan Z, Junkers T, Koo SPS, Lovestead TM, Davis TP, Stenzel MH, et al. Mapping photolysis product radical reactivities via soft ionization mass spectrometry in acrylate, methacrylate, and itaconate systems. Macromolecules. 2007;40:6820–33.
Willemse RXE, van Herk AM, Panchenko E, Junkers T, Buback M. PLP-ESR monitoring of midchain radicals in n-butyl acrylate polymerization. Macromolecules. 2005;38:5098–103.
Nikitin AN, Hutchinson RA. The effect of intramolecular transfer to polymer on stationary free radical polymerization of alkyl acrylates. Macromolecules. 2005;38:1581–90.
Junkers T, Koo SPS, Davis TP, Stenzel MH, Barner-Kowollik C. Mapping poly (butyl acrylate) product distributions by mass spectrometry in a wide temperature range: suppression of midchain radical side reactions. Macromolecules. 2007;40:8906–12.
Sanai Y, Kagami S, Kubota K. Cross-linking photopolymerization of monoacrylate initiated by benzophenone. J Polym Sci Part A. 2018;56:1545–53.
Nonaka H, Ouchi M, Kamigaito M, Sawamoto M. MALDI-TOF-MS analysis of rutherium(II)-mediated living radical polymerizations of methyl methacrylate, methyl acrylate, and styrene. Macromolecules. 2001;34:2083–8.
Farcet C, Belleney J, Charleux B, Pirri R. Structural characterization of nitroxide-terminated poly(n-butyl acrylate) prepared in bulk and miniemulsion polymerizations. Macromolecules. 2002;35:4912–8.
Matyjaszewski K, Nakagawa Y, Jasieczec CB. Polymerization of n-butyl acrylate by atom transfer radical polymerization. Remarkable effect of ethylene carbonate and other solvents. Macromolecules. 1998;31:1535–41.
Ligon SC, Husár B, Wutzel H, Holman R, Liska R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem Rev. 2014;114:557–89.
Pynaert R, Buguet J, Croutxé-Barghorn C, Moireau P, Allonas X. Effect of reactive oxygen species on the kinetics of free radical photopolymerization. Polym Chem. 2013;4:2475–9.
Sanai Y, Kagami S, Kubota K. Initiation and termination pathways in the photopolymerization of acrylate using methyl phenylglyoxylate as an initiator. Polym J. 2020;52:375–85.
Klems JP, Lippa KA, McGivern WS. Quantitative evidence for organic peroxy radical photochemistry at 254 nm. J Phys Chem A. 2015;119:344–51.
Buback M, Günzeler F, Russell GT, Vana P. Determination of the mode of termination in radical polymerization. Macromolecules 2009;42:652–62.
Harada T, Zetterlund PB, Yamada B. Preparation of macromonomers by copolymerization of methyl acrylate dimer involving β Fragmentation. J Polym Sci Part A. 2004;42:597–607.
Pitts MR, Harrison JR, Moody CJ. Indium metal as a reducing agent in organic synthesis. J Chem Soc Perkin Trans 1. 2001;1:955–77.
Gregg BT, Golden KC, Quinn JF. Indium(III)trifluoromethanesulfonate as a mild, efficient catalyst for the formation of acetals and ketals in the presence of acid sensitive functional groups. Tetrahedron. 2008;64:3287–95.
Sakai N, Moritaka K, Konakahara T. A novel approach to the practical synthesis of sulfides: an InBr3-Et3SiH catalytic system promoted the direct reductive sulfidation of acetals with disulfides. Eur J Org Chem. 2009;24:4123–7.
Iwasaki T, Maegawa Y, Hayashi Y, Ohshima T, Mashima K. Transesterification of various methyl esters under mild conditions catalyzed by tetranuclear zinc cluster. J Org Chem. 2008;73:5147–50.
Werner T, Barrett GM. Simple method for the preparation of esters from Grignard reagents and alkyl 1-imidazolecarboxylates. J Org Chem. 2006;71:4302–4.
Ghebreyessus KY, Angelici RJ. Isomerizing-hydroboration of the monounsaturated fatty acid ester methyl oleate. Organometallics. 2006;25:3040–4.
Hirano T, Yamada B. Macromonomer formation by sterically hindered radical polymerization of methyl acrylate trimer at high temperature. Polymer. 2003;44:347–54.
Acknowledgements
The authors thank Chisato Shimaoka (Base Technology Center, Toagosei Co., Ltd) for helping with the SEC measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sanai, Y., Kubota, K. Effect of UV-curing conditions on the polymer structures: a comparison between coating and adhesive. Polym J 52, 1153–1163 (2020). https://doi.org/10.1038/s41428-020-0347-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0347-4
This article is cited by
-
Influence of UV Polymerization Curing Conditions on Performance of Acrylic Pressure Sensitive Adhesives
Macromolecular Research (2021)