Abstract
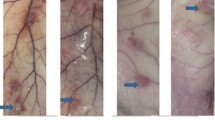
This paper focuses on the biocompatibility and biodegradation of PLA and a recently developed PLA/PCL blend containing an in vitro nontoxic compatibilizer based on a low-molecular-weight triblock copolymer derived from ε-caprolactone and tetrahydrofuran. The polymers were implanted subcutaneously in the lateral surface of the neck of horses. Physical examination, plasma fibrinogen (PF) analysis, infrared thermography (IT), mechanical nociceptive threshold (MNT) analysis, and ultrasonography were performed. After 24 weeks, the biomaterials were removed for histochemical analysis using hematoxylin-eosin (HE) and picrosirius-hematoxylin (PSH) staining. Scanning electron microscopy (SEM) was employed to determine changes in the surface morphology of the PLA and PLA/PCL blend. There were no clinical or PF changes. IT indicated a transient increase in cutaneous temperature (CT), while MNT decreased after the procedure in both the implanted groups. Ultrasonography revealed edema after the procedure and the loss of echogenicity of the polymers after implantation. Both polymers elicited a foreign body response under microscopic analysis. The PSH technique revealed a fibrotic reaction with collagen deposition around the polymers. SEM showed surface roughness, suggesting a biodegradation process. In conclusion, PLA and the PLA/PCL blend were biocompatible and biodegradable, with potential for use in equine medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings are available from the authors upon request.
References
Nampoothiri KM, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresour Technol. 2010;101:8493–501. https://doi.org/10.1016/j.biortech.2010.05.092
Theoret C. Physiology of wound healing. In: Theoret C, Schumacher J, editors. Equine wound management. Ames: John Wiley & Sons; 2017. p. 1–13.
FAO Food and Agriculture Organization, United Nations, Rome. 2017. http://www.fao.org/faostat/en/#data/QA. Accessed 09 Jan 2019.
Moreira RC, Graaf GMMVD, Pereira CA, Zoppa ALDVD. Mechanical evaluation of bone gap filled with rigid formulations castor oil polyurethane and chitosan in horses. Cienc Rural. 2016;46:2182–88. https://doi.org/10.1590/0103-8478cr20150838
Castro-Aguirre E, Iñiguez-Franco F, Samsudin H, Fang X, Auras R. Poly(lactic acid)—mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev. 2016;107:333–66. https://doi.org/10.1016/j.addr.2016.03.010
Xiao L, Wang B, Yang G, Gauthier M. Poly(lactic acid)-based biomaterials: synthesis, modification and applications. In: Ghista DN, editor. Biomedical Science, Engineering and Technology. London: InTech; 2012. p. 247–82.
Saini P, Arora M, Ravi Kumar MNV. Poly(lactic acid) blends in biomedical applications. Adv Drug Deliv Rev. 2016;107:47–59. https://doi.org/10.1016/j.addr.2016.06.014
Dias PP, Chinelatto MA. Effect of poly(ε-caprolactone-b-tetrahydrofuran) triblock copolymer concentration on morphological, thermal and mechanical properties of immiscible PLA/PCL blends. J Renew. 2019;7:129–38. https://doi.org/10.32604/jrm.2019.00037
Finotti PFM, Costa LC, Capote TSO, Scarel-Caminaga RM, Chinelatto MA. Immiscible poly(lactic acid)/poly(ε-caprolactone) for temporary implants: compatibility and cytotoxicity. J Mech Behav Biomed. 2017;68:155–62. https://doi.org/10.1016/j.jmbbm.2017.01.050
Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals. 6th ed. San Diego: Elsevier; 2008.
Clauss A. Gerinnugsphysiologische schnellmethode zur bestimmung des fibrinogens. Acta Haematol. 1957;17:237–46. https://doi.org/10.1159/000205234
Söbbeler FJ, Kästner SBR. Effects of transdermal lidocaine or lidocaine with prilocaine or tetracaine on mechanical superficial sensation and nociceptive termal thresholds in horses. Vet Anaesth Analg. 2018;45:227–33. https://doi.org/10.1016/j.vaa.2017.10.003
De Jong WH, Bergsma JE, Robinson JE, Bos RRM. Tissue response to partially in vitro predegraded poly-L-lactide implants. Biomaterials. 2005;26:1781–91. https://doi.org/10.1016/j.biomaterials.2004.06.026
Byars TD, Gonda KC. Equine history, physical examination, records, and recognizing abuse or neglect in patients. In: Smith BP, editor. Large animal internal medicine. Riverport Lane: Elsevier; 2015. p. 13–20.
Bundgaard L, Sørensen MA, Nilson T, Salling E, Jacobsen S. Evaluation of systemic and local inflammatory parameters and manifestations of pain in an equine experimental wound model. J Equine Vet Sci. 2018;68:81–7. https://doi.org/10.1016/j.jevs.2018.05.219
Hooijberg EH, van den Hoven R, Tichy A, Schwendenwein I. Diagnostic and predictive capability of routine laboratory tests for the diagnosis and staging of equine inflammatory disease. J Vet Intern. 2014;28:1587–93. https://doi.org/10.1111/jvim.12404
Johns JL. Alterations in blood proteins. In: Smith BP, editors. Large animal internal medicine. Riverport Lane: Elsevier; 2015. p. 386–92.
Jacobsen S, Nielsen JV, Kjelgaard-Hansen M, Toelboeli T, Fjeldborg J, Halling-Thomsen M, Martinussen T, Thoefner MB. Acute phase response to surgery of varying intensity in horses: a preliminary study. Vet Surg. 2009;38:762–69. https://doi.org/10.1111/j.1532-950X.2009.00564.x
Redaelli V, Bergero D, Zucca E, Ferrucci F, Costa LN, Crosta L, Luzi F. Use of thermography techniques in equines: principles and applications. J Equine Vet Sci 2014;34:345–50. https://doi.org/10.1016/j.jevs.2013.07.007
McManus C, Tanure CB, Peripolli V, Seixas L, Fischer V, Gabbi AM, Menegassi SRO, Stumpf MT, Kolling GJ, Dias E, Costa JBG Jr. Infrared thermography in animal production: an overview. Comput Electron Agr. 2016;123:10–1. https://doi.org/10.1016/j.compag.2016.01.027
Nóbrega FS, Ferreira MP, Facó LL, Selim MB, De Zoppa ALV. Uso da termografia para avaliação da resposta tecidual após implante de polímero a base de poliuretana de mamona em osso III metacarpiano de equinos. Acta Sci Vet. 2014;42:1–5.
Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14:1255–69. https://doi.org/10.1016/j.jpain.2013.06.008
Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Ski Res Technol. 2005;11:221–35. https://doi.org/10.1111/j.0909-725X.2005.00151.x
Höglund OV, Hagman R, Olsson K, Carlsson C, Södersten F, Lagerstedt AS. Ligation of the ovarian pedicles in dogs with a resorbable self-locking device—a long-term follow-up study. J Biomater Appl. 2013;27:961–6. https://doi.org/10.1177/0885328211431018
Höglund OV, Ingman J, Södersten F, Hansson K, Borg N, Lagerstedt AS. Ligation of the spermatic cord in dogs with a self-locking device of a resorbable polyglycolic based co-polymer—feasibility and long-term follow-up study. BMC Res Notes. 2014;7:825–31. https://doi.org/10.1186/1756-0500-7-825
Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32:762–98. https://doi.org/10.1016/j.progpolymsci.2007.05.017
ISO. Biological evaluation of medical devices. Part 6: tests for local effects after implantation. Geneva, Switzerland: International Organization for Standardization; 1994.
Kastellorizios M, Papadimitrakopoulos F, Burgess DJ. Prevention of foreign body reaction in a pre-clinical large animal model. J Control Release 2015;202:101–7. https://doi.org/10.1016/j.jconrel.2015.01.038
Ciambelli GS, Perez MO, Siqueira GV, Candella MA, Motta AC, Duarte MAT, Alberto-Rincon MC, Duek EAR. Characterization of poly (L-co-D, L lactic acid) and a study of polymer-tissue interaction in subcutaneous implants in Wistar rats. Mater Res 2013;16:28–37. https://doi.org/10.1590/S1516-14392012005000146
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. https://doi.org/10.1016/j.smim.2007.11.004
Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006. https://doi.org/10.1088/1748-6041/1/1/R01
Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12:188–96. https://doi.org/10.1208/s12248-010-9175-3
Nyska A, Schiffenbauer YS, Brami CT, Maronpot RR, Ramot Y. Histopathology of biodegradable polymers: challenges in interpretation and the use of a novel compact MRI for biocompatibility evaluation. Polym Advan Technol. 2014;25:461–7. https://doi.org/10.1002/pat.3238
Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacík I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. 2015;14:643–52. https://doi.org/10.1038/NMAT4290
Fournier E, Passirani C, Montero-Menei CN, Benoit JP. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003;24:3311–31. https://doi.org/10.1016/S0142-9612(03)00161-3
Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009;30:2956–65. https://doi.org/10.1016/j.biomaterials.2009.02.006
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;290602:1–19. https://doi.org/10.1155/2011/290602
Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517–24. https://doi.org/10.1002/jbm.820291208
Vidal BC, Mello MLS, Pimentel ER. Polarization microscopy and microspectrophotometry of Sirius Red, Picrosirius and Chlorantine Fast Red aggregates and of their complexes with collagen. Histochem J. 1982;14:857–78.
Ribeiro JF, Anjos EHM, Mello MLS, Vidal BC. Skin collagen fiber molecular order: a pattern of distributional fiber orientation as assessed by optical anisotropy and image analysis. Plos ONE 2013;8:1–9. https://doi.org/10.1371/journal.pone.0054724
Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–53. https://doi.org/10.1016/j.biomaterials.2008.04.023
Acknowledgements
This work was funded by The São Paulo Research Foundation (FAPESP, process no. 2017/10959-4 and process no. 2015/26738-1) and the National Council for Scientific and Technological Development (CNPq, process no. 132700/2017-4). Also, this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. We thank Professor Áureo Evangelista Santana and the Clinical Pathology Laboratory of the Veterinary Hospital (FCAV/UNESP) for their excellent technical assistance, and Professor Fernando José Zara for his collaboration in conducting the scanning electron microscopy analysis. We also thank the Graduate Course Program in Veterinary Medicine (FCAV/UNESP) and the Department of Animal Morphology and Physiology (FCAV/UNESP) for the academic support. This work is part of the requirements to obtain a Ph.D. degree by Júlia Ribeiro Garcia de Carvalho in the Graduate Course in Veterinary Medicine (FCAV/UNESP), SP, Brazil.
Author information
Authors and Affiliations
Contributions
GCF and JRGC designed the study. GCF supervised the study. PPD and MAC developed and supplied the polymers used in the study, analyzed data concerning polymer biodegradation and wrote these results in the paper. JRGC, GC, and MLA performed the experiment and collected the data. JRGC, GC, ROV, SRT, and GCF analyzed the data and interpreted the results. GTP and GCF performed the statistical analyses. JRGC, GC, MLA, and GCF drafted the paper. ROV, SRT, PAC, PPD, MAC and GCF aided in planning and provided critical readings of the paper. JRGC and GCF wrote and finalized the article. All authors are aware of the content and have read and edited the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The study followed the Ethical Principles in Animal Experimentation adopted by the Brazilian College of Animal Experimentation and was approved and supervised by the institutional animal use and care committee (006548/17).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carvalho, J.R.G., Conde, G., Antonioli, M.L. et al. Biocompatibility and biodegradation of poly(lactic acid) (PLA) and an immiscible PLA/poly(ε-caprolactone) (PCL) blend compatibilized by poly(ε-caprolactone-b-tetrahydrofuran) implanted in horses. Polym J 52, 629–643 (2020). https://doi.org/10.1038/s41428-020-0308-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0308-y
This article is cited by
-
A preliminary investigation of the subcutaneous tissue reaction to a 3D printed polydioxanone device in horses
Acta Veterinaria Scandinavica (2023)
-
PCL-based hydrophobic chains grafted with two PEG-based hydrophilic branches: fluorescence and dynamic light scattering studies
Journal of Polymer Research (2023)
-
Biodegradable aliphatic poly(carbonate-co-ester)s containing biobased unsaturated double bonds: synthesis and structure-property relationships
Polymer Journal (2022)
-
Isolating motile sperm cell sorting using biocompatible electrospun membranes
Scientific Reports (2022)
-
Near-ambient pressure X-ray photoelectron spectroscopy for a bioinert polymer film at a water interface
Polymer Journal (2021)