Abstract
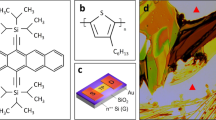
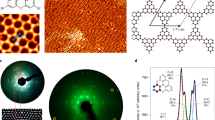
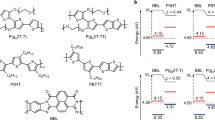
Liquid-crystalline (LC) π-conjugated polymers are an emerging class of semiconducting materials owing to their promising performance in organic field-effect transistors (OFETs). Little is known, however, about the relationship between LC nature and charge carrier mobility. In this paper, we focus on a thiophene-based p-type semiconducting polymer, PC12TV12T, containing thienylene–vinylene–thienylene (TVT) units, and report a systematic investigation of its thermotropic LC properties, self-organized structures in bulk and thin films, as well as charge transport properties in OFETs. We found that thermal annealing at LC temperatures (99–170 °C) strongly enhanced OFET performance, leading to field-effect hole mobilities as high as 0.37 cm2 V−1 s−1, comparable to that of amorphous silicon. By virtue of its thermoplasticity, the TVT-based polymer can also be processed into fine semiconducting microfibers, which can even function as a p-type active channel for charge transport. This bottom-up technology utilizing the LC nature enables cost-effective and energy-efficient manufacture of optoelectronic devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Garnier F, Hajlaoui R, Yassar A, Srivastava P. All-polymer field-effect transistor realized by printing techniquies. Science. 1994;265:1684–6.
Bao Z, Dodabalapur A, Lovinger AJ. Soluble and processable regioregular poly(3-hexylthiophene) for thin film field-effect transistor applications with high mobility. Appl Phys Lett. 1996;69:4108–10.
Sirringhaus H, Brown PJ, Friend RH, Nielsen MM, Bechgaard K, Langeveld-Voss BMW, et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature. 1999;401:685–8.
Sirringhaus H, Kawase T, Friend RH, Shimoda T, Inbasekaran M, Wu W, et al. High-resolution inkjet printing of all-polymer transistor circuits. Science. 2000;290:2123–6.
Arias AC, MacKenzie JD, McCulloch I, Rivnay J, Salleo A. Materials and applications for large area electronics: solution-based approaches. Chem Rev. 2010;110:3–24.
Facchetti A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem Mater. 2011;23:733–58.
Wang C, Dong H, Hu W, Liu Y, Zhu D. Semiconducting π-conjugated systems in field-effect transistors: a material odyssey of organic electronics. Chem Rev. 2012;112:2208–67.
Holliday S, Donaghey JE, McCulloch I. Advances in charge carrier mobilities of semiconducting polymers used in organic transistors. Chem Mater. 2014;26:647–63.
Sirringhaus H. 25th Anniversary article: organic field-effect transistors: the path beyond amorphous silicon. Chem Mater. 2014;26:1319–35.
Luo H, Liu Z, Zhang D. Conjugated D–A terpolymers for organic field-effect transistors and solar cells. Polym J. 2018;50:21–31.
Diao Y, Shaw L, Bao Z, Mannsfeld SCB. Morphology control strategies for solution-processed organic semiconductor thin films. Energy Environ Sci. 2014;7:2145–59.
Khim D, Luzio A, Bonacchini GE, Pace G, Lee MJ, Noh YY, et al. Uniaxial alignment of conjugated polymer films for high-performance organic field-effect transistors. Adv Mater. 2018;30:1–34.
Brandão L, Viana J, Bucknall DG, Bernardo G. Solventless processing of conjugated polymers–A review. Synth Met. 2014;197:23–33.
Kato T, Uchida J, Ichikawa T, Sakamoto T. Functional liquid crystals towards the next generation of materials. Angew Chem Int Ed. 2018;57:4355–71.
Kato T, Yoshio M, Ichikawa T, Soberats B, Ohno H, Funahashi M. Transport of ions and electrons in nanostructured liquid crystals. Nat Rev Mater. 2017;2:17001.
Kato T, Uchida J, Ichikawa T, Soberats B. Functional liquid-crystalline polymers and supramolecular liquid crystals. Polym J. 2018;50:149–66.
Fleischmann E-K, Zentel R. Liquid-crystalline ordering as a concept in materials science: from semiconductors to stimuli-responsive devices. Angew Chem Int Ed. 2013;52:8810–27.
O’Neill M, Kelly SM. Ordered materials for organic electronics and photonics. Adv Mater. 2011;23:566–84.
Funahashi M. Nanostructured liquid-crystalline semiconductors—a new approach to soft matter electronics. J Mater Chem C. 2014;2:7451–9.
Funahashi M. Development of liquid-crystalline semiconductors with high carrier mobilities and their application to thin-film transistors. Polym J. 2009;41:459–69.
Iino H, Hanna J. Liquid crystalline organic semiconductors for organic transistor applications. Polym J. 2017;49:23–30.
McCullough RD. The chemistry of conducting polythiophenes. Adv Mater. 1998;10:93–116.
Ho V, Boudouris BW, Segalman RA. Tuning polytiophene crystallization through systematic side chain functionalization. Macromolecules. 2010;43:7895–9.
McCulloch I, Heeney M, Bailey C, Genevicius K, MacDonald I, Shkunov M, et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat Mater. 2006;5:328–33.
Hamadani BH, Gundlach DJ, McCulloch I, Heeney M. Undoped polythiophene field-effect transistors with mobility of 1 cm2 V−1 s−1. Appl Phys Lett. 2007;91:1–4.
Umeda T, Kumaki D, Tokito S. Surface-energy-dependent field-effect mobilities up to 1 cm2 V−1 s−1 for polymer thin-film transistor. J Appl Phys. 2009;105:024516.
Ong BS, Wu Y, Liu P, Gardner S. High-performance semiconducting polythiophenes for organic thin-film transistors. J Am Chem Soc. 2004;126:3378–9.
Zhao N, Botton GA, Zhu S, Duft A, Ong BS, Wu Y, et al. Microscopic studies on liquid crystal poly(3,3‴-dialkylquaterthiophene) semiconductor. Macromolecules. 2004;37:8307–12.
Wu Y, Liu P, Ong BS, Srikumar T, Zhao N, Botton G, et al. Controlled orientation of liquid-crystalline polythiophene semiconductors for high-performance organic thin-film transistors. Appl Phys Lett. 2005;86:142102.
Kim DH, Lee B-L, Moon H, Kang HM, Jeong EJ, Oark J-I, et al. J Am Chem Soc. 2009;131:6124–32.
Kim J, Lim B, Baeg KJ, Noh YY, Khim D, Jeong HG, et al. Highly soluble poly(thienylenevinylene) derivatives with charge-carrier mobility exceeding 1 cm2 V−1 s−1. Chem Mater. 2011;23:4663–5.
Lim B, Baeg K-J, Jeong H-G, Jo J, Kim H, Park J-W, et al. A new poly(thienylenevinylene) derivative with high mobility and oxidative stability for organic thin-film transistors and solar cells. Adv Mater. 2009;21:2808–14.
Jang S-Y, Lim B, Yu B-K, Kim J, Baeg K-J, Khim D, et al. Synthesis and characterization of low-band-gap poly(thienylenevinylene) derivatives for polymer solar cells. J Mater Chem. 2011;21:11822–30.
Speros JC, Martinez H, Paulsen BD, White SP, Bonifas AD, Goff PC, et al. Effects of olefin content and alkyl chain placement on optoelectronic and morphological properties in poly(thienylene vinylenes). Macromolecules. 2013;46:5184–94.
Fei Z, Pattanasattayavong P, Han Y, Schroeder BC, Yan F, Kline RJ, et al. Influence of side-chain regiochemistry on the transistor performance of high-mobility, all-donor polymers. J Am Chem Soc. 2014;136:15154–7.
Jang SY, Kim I-B, Kim J, Khim D, Jung E, Kang B, et al. New donor–donor type copolymers with rigid and coplanar structures for high-mobility organic field-effect transistors. Chem Mater. 2014;26:6907–10.
Chen H, Guo Y, Yu G, Zhao Y, Zhang J, Gao D, et al. Highly π-extended copolymers with diketopyrrolopyrrole moieties for high-performance field-effect transistors. Adv Mater. 2012;24:4618–22.
Kim R, Amegadze PSK, Kang I, Yun H-J, Noh Y-Y, Kwon S-K, et al. High-mobility air-stable naphthalene diimide-based copolymer containing extended π-conjugation for n-channel organic field effect transistors. Adv Funct Mater. 2013;23:5719–27.
Kim J, Baeg K-J, Khim D, James DT, Kim J-S, Lim B, et al. Optimal ambipolar charge transport of thienylenevinylene-based polymer semiconductors by changes in conformation for high-performance organic thin film transistors and inverters. Chem Mater. 2013;25:1572–83.
Chen H, Guo Y, Mao Z, Yu G, Huang J, Zhao Y, et al. Naphthalenediimide-based copolymers incorporating vinyl-linkages for high-performance ambipolar field-effect transistors and complementary-like inverters under air. Chem Mater. 2013;25:4835–4835.
Lim D-H, Jang S-Y, Kang M, Lee S, Kim Y-A, Heo Y-J, et al. A systematic study on molecular planarity and D–A conformation in thiazolothiazole- and thienylenevinylene-based copolymers for organic field-effect transistors. J Mater Chem C. 2017;5:10126–32.
Kang S-H, Lee HR, Dutta GK, Lee J, Oh JH, Yang C. A role of side-chain regiochemistry of thienylene–vinylene–thienylene (TVT) in the transistor performance of isomeric polymers. Macromolecules. 2017;50:884–90.
Sandberg HGO, Frey GL, Shkunov MN, Sirringhaus H, Friend RH, Nielsen MM, et al. Ultrathin regioregular poly(3-hexyl thiophene) field-effect transistors. Langmuir. 2002;18:10176–82.
Wang G, Swensen J, Moses D, Heeger AJ. Increased mobility from regioregular poly(3-hexylthiophene) field-effect transistors. J Appl Phys. 2003;93:6137–41.
Tsao HN, Cho D, Andreasen JW, Rouhanipour A, Breiby DW, Pisula W, et al. The influence of morphology on high-performance polymer field-effect transistors. Adv Mater. 2009;21:209–12.
Wang S, Kiersnowski A, Pisula W, Müllen K. Microstructure evolution and device performance in solution-processed polymeric field-effect transistors: the key role of the first monolayer. J Am Chem Soc. 2012;134:4015–8.
Mori T, Oyama T, Komiyama H, Yasuda T. Solution-grown unidirectionally oriented crystalline thin films of a U-shaped thienoacene-based semiconductor for high-performance organic field-effect transistors. J Mater Chem C. 2017;5:5872–6.
Oyama T, Mori T, Hashimoto T, Kamiya M, Ichikawa T, Komiyama H, et al. High-mobility regioisomeric thieno[f,f’]bis[1]benzothiophenes: remarkable effect of syn/anti thiophene configuration on optoelectronic properties, self-organization, and charge-transport functions in organic transistors. Adv Electron Mater. 2018;4:1700390.
Acknowledgements
This work was partially supported by KAKENHI (Grant Nos. JP18H02048 (TY), JP18J22297 (TM), and JP16K21218 (HK)) from the Japan Society for the Promotion of Science (JSPS) and the Amano Institute of Technology (TY). TM is grateful for the financial support from the JSPS Research Fellowship. The authors acknowledge the support of the Cooperative Research Program “Network Joint Research Center for Materials and Devices”. The GIXD experiments were performed at the BL40-B2 beamline in SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2017A1119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mori, T., Komiyama, H., Ichikawa, T. et al. A liquid-crystalline semiconducting polymer based on thienylene–vinylene–thienylene: Enhanced hole mobilities by mesomorphic molecular ordering and thermoplastic shape-deformable characteristics. Polym J 52, 313–321 (2020). https://doi.org/10.1038/s41428-019-0282-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-019-0282-4