Abstract
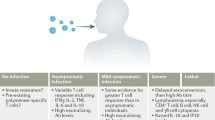
SARS-CoV-2 infections manifest with a broad spectrum of presentations, ranging from asymptomatic infections to severe pneumonia and fatal outcomes. This review centers on asymptomatic infections, a widely reported phenomenon that has substantially contributed to the rapid spread of the pandemic. In such asymptomatic infections, we focus on the role of innate, humoral, and cellular immunity. Notably, asymptomatic infections are characterized by an early and robust innate immune response, particularly a swift type 1 IFN reaction, alongside a rapid and broad induction of SARS-CoV-2-specific T cells. Often, antibody levels tend to be lower or undetectable after asymptomatic infections, suggesting that the rapid control of viral replication by innate and cellular responses might impede the full triggering of humoral immunity. Even if antibody levels are present in the early convalescent phase, they wane rapidly below serological detection limits, particularly following asymptomatic infection. Consequently, prevalence studies reliant solely on serological assays likely underestimate the extent of community exposure to the virus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Christian MD, Poutanen SM. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–27.
Gandhi RT, Lynch JB. Mild or Moderate Covid-19. N. Engl J Med. 2020;383:1757–66.
Lee S, Kim T. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the republic of Korea. JAMA Intern Med. 2020;180:1447–52.
Han H, Xu Z. Descriptive, retrospective study of the clinical characteristics of asymptomatic COVID-19 patients. mSphere. 2020;5:e00922–20.
Johansson MA, Quandelacy TM. SARS-CoV-2 transmission from people without COVID-19 Symptoms. JAMA Netw Open. 2021;4:e2035057.
Buitrago-Garcia D, Ipekci AM. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: Update of a living systematic review and meta-analysis. PLoS Med. 2022;19:e1003987.
Tsikala Vafea M, Atalla E. Chest CT findings in asymptomatic cases with COVID-19: a systematic review and meta-analysis. Clin Radio. 2020;75:876.e833–876 e839.
Mizumoto K, Kagaya K. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eur Surveill. 2020;25:2000180.
Sakurai A, Sasaki T. Natural History of Asymptomatic SARS-CoV-2 Infection. N. Engl J Med. 2020;383:885–6.
Arons MM, Hatfield KM. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl J Med. 2020;382:2081–90.
Kasper MR, Geibe JR. An Outbreak of Covid-19 on an Aircraft Carrier. N. Engl J Med. 2020;383:2417–26.
Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann Intern Med. 2021;174:286–7.
Van Vinh Chau N, Lam VT. The Natural History and Transmission Potential of Asymptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clin Infect Dis. 2020;71:2679–87.
Cevik M, Tate M. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22.
Gunatilaka AB, Marco N. Viral Burden and Clearance in Asymptomatic COVID-19 Patients. Open Forum Infect Dis. 2022;9:ofac126.
He Z, Ren L. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397:1075–84.
Fauci AS, Lane HC. Covid-19 - Navigating the Uncharted. N. Engl J Med. 2020;382:1268–9.
Bergeri I, Whelan MG. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022;19:e1004107.
Clarke KEN, Jones JM. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies - United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:606–8.
Ng TC, Cheng HY. Comparison of Estimated Effectiveness of Case-Based and Population-Based Interventions on COVID-19 Containment in Taiwan. JAMA Intern Med. 2021;181:913–21.
Iezadi S, Gholipour K. Effectiveness of non-pharmaceutical public health interventions against COVID-19: A systematic review and meta-analysis. PLoS One. 2021;16:e0260371.
Hoze N, Paireau J. Monitoring the proportion of the population infected by SARS-CoV-2 using age-stratified hospitalisation and serological data: a modelling study. Lancet Public Health. 2021;6:e408–e415.
Bobrovitz N, Arora RK. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLoS One. 2021;16:e0252617.
Jones JM, Manrique IM. Estimates of SARS-CoV-2 Seroprevalence and Incidence of Primary SARS-CoV-2 Infections Among Blood Donors, by COVID-19 Vaccination Status - United States, April 2021-September 2022. MMWR Morb Mortal Wkly Rep. 2023;72:601–5.
Crotty S. Hybrid immunity. Science. 2021;372:1392–3.
Altarawneh HN, Chemaitelly H. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl J Med. 2022;387:21–34.
Bobrovitz N, Ware H. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23:556–67.
Gandhi M. Immunity Against the Omicron Variant From Vaccination, Recovery, or Both. Clin Infect Dis. 2022;75:e672–e674.
Takahashi S, Peluso MJ. SARS-CoV-2 Serology Across Scales: A Framework for Unbiased Estimation of Cumulative Incidence Incorporating Antibody Kinetics and Epidemic Recency. Am J Epidemiol. 2023;192:1562–75.
Markov PV, Ghafari M. The evolution of SARS-CoV-2. Nat Rev Microbiol. 2023;21:361–79.
Li R, Pei S. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368:489–93.
Team C-F. Variation in the COVID-19 infection-fatality ratio by age, time, and geography during the pre-vaccine era: a systematic analysis. Lancet. 2022;399:1469-88.
Letizia AG, Smith DR. Viable virus shedding during SARS-CoV-2 reinfection. Lancet Respir Med. 2021;9:e56–e57.
Hall VJ, Foulkes S. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397:1459–69.
Yan X, Han X. Clinical Characteristics and Prognosis of 218 Patients With COVID-19: A Retrospective Study Based on Clinical Classification. Front Med (Lausanne). 2020;7:485.
Hurst JH, Heston SM. Severe Acute Respiratory Syndrome Coronavirus 2 Infections Among Children in the Biospecimens from Respiratory Virus-Exposed Kids (BRAVE Kids) Study. Clin Infect Dis. 2021;73:e2875–e2882.
Li J, Thoon KC. Comparative Analysis of Symptomatic and Asymptomatic SARS-CoV-2 Infection in Children. Ann Acad Med Singap. 2020;49:530–7.
Wang B, Andraweera P. Asymptomatic SARS-CoV-2 Infection by Age: A Global Systematic Review and Meta-analysis. Pediatr Infect Dis J. 2023;42:232–9.
Chen X, Huang Z. Ratio of asymptomatic COVID-19 cases among ascertained SARS-CoV-2 infections in different regions and population groups in 2020: a systematic review and meta-analysis including 130 123 infections from 241 studies. BMJ Open. 2021;11:e049752.
Baden LR, El Sahly HM. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl J Med. 2021;384:403–16.
Sadoff J, Gray G. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl J Med. 2021;384:2187–201.
Ali K, Berman G. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl J Med. 2021;385:2241–51.
Takuva S, Takalani A. Thromboembolic Events in the South African Ad26.COV2.S Vaccine Study. N. Engl J Med. 2021;385:570–1.
Garrett N, Tapley A. High Asymptomatic Carriage With the Omicron Variant in South Africa. Clin Infect Dis. 2022;75:e289–e292.
Carabelli AM, Peacock TP. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21:162–77.
Lumley SF, O’Donnell D. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl J Med. 2021;384:533–40.
Bergwerk M, Gonen T. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl J Med. 2021;385:1474–84.
Bortolotti D, Gentili V. TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection. Microorganisms. 2021;9:1820.
Hadjadj J, Yatim N. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.
Cobat A, Zhang Q. Human Genomics of COVID-19 Pneumonia: Contributions of Rare and Common Variants. Annu Rev Biomed Data Sci. 2023;6:465–86.
Bastard P, Rosen LB. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370:eabd4585.
Chandran A, Rosenheim J. Rapid synchronous type 1 IFN and virus-specific T cell responses characterize first wave non-severe SARS-CoV-2 infections. Cell Rep. Med. 2022;3:100557.
Swadling L, Diniz MO. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2022;601:110–7.
Zhao XN, You Y. Single-cell immune profiling reveals distinct immune response in asymptomatic COVID-19 patients. Signal Transduct Target Ther. 2021;6:342.
Long QX, Tang XJ. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–4.
Soares-Schanoski A, Sauerwald N. Asymptomatic SARS-CoV-2 Infection Is Associated With Higher Levels of Serum IL-17C, Matrix Metalloproteinase 10 and Fibroblast Growth Factors Than Mild Symptomatic COVID-19. Front Immunol. 2022;13:821730.
Cusenza F, Davino G. Silence of the Lambs: The Immunological and Molecular Mechanisms of COVID-19 in Children in Comparison with Adults. Microorganisms. 2021;9:330.
Wimmers F, Burrell AR. Multi-omics analysis of mucosal and systemic immunity to SARS-CoV-2 after birth. Cell. 2023;186:4632–51.e4623.
Agrati C, Carsetti R. The immune response as a double-edged sword: The lesson learnt during the COVID-19 pandemic. Immunology. 2022;167:287–302.
Gilbert PB, Montefiori DC. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50.
Weinreich DM, Sivapalasingam S. REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. N. Engl J Med. 2021;385:e81.
Weinreich DM, Sivapalasingam S. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl J Med. 2021;384:238–51.
O’Brien MP, Forleo-Neto E. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N. Engl J Med. 2021;385:1184–95.
Reynolds CJ, Swadling L. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol. 2020;5:eabf3698.
Dorigatti I, Lavezzo E. SARS-CoV-2 antibody dynamics and transmission from community-wide serological testing in the Italian municipality of Vo’. Nat Commun. 2021;12:4383.
Chia WN, Zhu F. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249.
Chen X, Pan Z. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther. 2020;5:180.
Jiang C, Wang Y. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Transl Immunol. 2020;9:e1182.
Ibarrondo FJ, Fulcher JA. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl J Med. 2020;383:1085–7.
Trinite B, Tarres-Freixas F. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci Rep. 2021;11:2608.
Nyagwange J, Ndwiga L. Epidemiology of COVID-19 infections on routine polymerase chain reaction (PCR) and serology testing in Coastal Kenya. Wellcome Open Res. 2022;7:69.
Fenwick C, Croxatto A. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol. 2021;95:e01828–20.
Wang Z, Yang X. SARS-CoV-2-specific CD4(+) T cells are associated with long-term persistence of neutralizing antibodies. Signal Transduct Target Ther. 2022;7:132.
Tan AT, Linster M. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728.
Menges D, Zens KD. Heterogenous humoral and cellular immune responses with distinct trajectories post-SARS-CoV-2 infection in a population-based cohort. Nat Commun. 2022;13:4855.
Russell MW, Mestecky J. Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front Immunol. 2022;13:957107.
Froberg J, Gillard J. SARS-CoV-2 mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat Commun. 2021;12:5621.
Chan RWY, Chan KCC. Mucosal Antibody Response to SARS-CoV-2 in Paediatric and Adult Patients: A Longitudinal Study. Pathogens. 2022;11:397.
Thomas AC, Oliver E. Evaluation and deployment of isotype-specific salivary antibody assays for detecting previous SARS-CoV-2 infection in children and adults. Commun Med (Lond). 2023;3:37.
Grifoni A, Weiskopf D. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–501.e1415.
Weiskopf D, Schmitz KS. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5:eabd2071.
Braun J, Loyal L. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–4.
Snyder TM, Gittelman RM. Magnitude and Dynamics of the T-Cell Response to SARS-CoV-2 Infection at Both Individual and Population Levels. medRxiv. https://doi.org/10.1101/2020.07.31.20165647 2020.
Le Bert N, Tan AT. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–62.
Meckiff BJ, Ramirez-Suastegui C. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell. 2020;183:1340–53.e1316.
Bacher P, Rosati E. Low-Avidity CD4(+) T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity. 2020;53:1258–71.e1255.
Peng Y, Mentzer AJ. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–45.
Cervia C, Nilsson J. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2021;147:545–57.e549.
Sekine T, Perez-Potti A, Robust T. Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020;183:158–68.e114.
Schwarzkopf S, Krawczyk A. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg Infect Dis. 2021;27:1.
Rydyznski Moderbacher C, Ramirez SI. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996–1012.e1019.
Le Bert N, Clapham HE. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218:e20202617.
Norton NJ, Holder KA. Cellular Immune Responses to SARS-CoV-2 in Exposed Seronegative Individuals. Viruses. 2023;15:996.
de Ruiter K, Jochems SP. Helminth infections drive heterogeneity in human type 2 and regulatory cells. Sci Transl Med. 2020;12:eaaw3703.
Mbow M, de Jong SE. Changes in immunological profile as a function of urbanization and lifestyle. Immunology. 2014;143:569–77.
Samandari T, Ongalo J. Prevalence and functional profile of SARS-CoV-2 T cells in asymptomatic Kenyan adults. J Clin Invest. 2023;133:e170011.
Tarke A, Potesta M. Early and Polyantigenic CD4 T Cell Responses Correlate with Mild Disease in Acute COVID-19 Donors. Int J Mol Sci. 2022;23:7155.
Augusto DG, Murdolo LD. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection. Nature. 2023;620:128–36.
Marchal A, Cirulli ET. Lack of association between HLA and asymptomatic SARS-CoV-2 infection. medRxiv. https://doi.org/10.1101/2023.12.06.23299623 2023.
Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–9.
Uddbäck I, Michalets SE. Prevention of respiratory virus transmission by resident memory CD8(+) T cells. Nature. https://doi.org/10.1038/s41586-023-06937-1 2023.
Che XY, Di B. A patient with asymptomatic severe acute respiratory syndrome (SARS) and antigenemia from the 2003-4 community outbreak of SARS in Guangzhou, China. Clin Infect Dis. 2006;43:e1–5.
Wilder-Smith A, Teleman MD. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11:1142–5.
Ho KY, Singh KS. Mild illness associated with severe acute respiratory syndrome coronavirus infection: lessons from a prospective seroepidemiologic study of health-care workers in a teaching hospital in Singapore. J Infect Dis. 2004;189:642–7.
Cao WC, Liu W. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl J Med. 2007;357:1162–3.
Wu LP, Wang NC. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13:1562–4.
Ng OW, Chia A. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34:2008–14.
Alhabbab RY, Algaissi A. Middle East Respiratory Syndrome Coronavirus Infection Elicits Long-lasting Specific Antibody, T and B Cell Immune Responses in Recovered Individuals. Clin Infect Dis. 2023;76:e308–e318.
Shin HS, Kim Y. Immune Responses to Middle East Respiratory Syndrome Coronavirus During the Acute and Convalescent Phases of Human Infection. Clin Infect Dis. 2019;68:984–92.
Reusken CB, Farag EA. Occupational Exposure to Dromedaries and Risk for MERS-CoV Infection, Qatar, 2013-4. Emerg Infect Dis. 2015;21:1422–5.
Alshukairi AN, Zheng J. High Prevalence of MERS-CoV Infection in Camel Workers in Saudi Arabia. mBio. 2018;9:e01985–18.
Mok CKP, Zhu A. T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: an observational cohort study. Lancet Infect Dis. 2021;21:385–95.
Mateus J, Dan JM. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science. 2021;374:eabj9853.
Garcia-Jimenez AF, Caceres-Martell Y. Cross-reactive cellular, but not humoral, immunity is detected between OC43 and SARS-CoV-2 NPs in people not infected with SARS-CoV-2: Possible role of cT(FH) cells. J Leukoc Biol. 2022;112:339–46.
Kundu R, Narean JS. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat Commun. 2022;13:80.
Murray SM, Ansari AM. The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat Rev Immunol. 2023;23:304–16.
Zhou P, Yang XL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3.
Tan AT, Lim JM. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J Clin Invest. 2021;131:e152379.
Collie S, Champion J. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl J Med. 2022;386:494–6.
Kirsebom FCM, Andrews N. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect Dis. 2022;22:931–3.
Tang P, Hasan MR. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27:2136–43.
Kalimuddin S, Tham CYL. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med (N. Y). 2021;2:682–8.e684.
Zhang B, Upadhyay R. Multimodal single-cell datasets characterize antigen-specific CD8(+) T cells across SARS-CoV-2 vaccination and infection. Nat Immunol. 2023;24:1725–34.
Tarke A, Coelho CH. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–59.e811.
GeurtsvanKessel CH, Geers D. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022;7:eabo2202.
Gao Y, Cai C. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat Med. 2022;28:472–6.
De Marco L, D’Orso S. Assessment of T-cell Reactivity to the SARS-CoV-2 Omicron Variant by Immunized Individuals. JAMA Netw Open. 2022;5:e2210871.
Naranbhai V, Nathan A. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell. 2022;185:1041–51.e1046.
Painter MM, Johnston TS. Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nat Immunol. 2023;24:1711–24.
Lim JME, Tan AT. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J Exp Med. 2022;219:e20220780.
Roukens AHE, Pothast CR. Prolonged activation of nasal immune cell populations and development of tissue-resident SARS-CoV-2-specific CD8(+) T cell responses following COVID-19. Nat Immunol. 2022;23:23–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
NLB declares a patent application for a method to monitor SARS-CoV-2-specific T cells in biological samples pending, and she is a co-founder of T Cell Diagnostics (TCD) Ltd. TS declares no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Bert, N., Samandari, T. Silent battles: immune responses in asymptomatic SARS-CoV-2 infection. Cell Mol Immunol 21, 159–170 (2024). https://doi.org/10.1038/s41423-024-01127-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-024-01127-z