Abstract
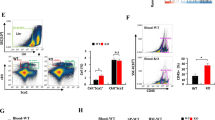
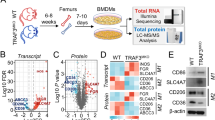
Macrophages are highly plastic cells that differentially regulate multiple pathological conditions, including cancer and autoimmune diseases. In response to various stimuli, macrophages activate different intrinsic signaling pathways and polarize into distinct macrophage subsets. We aimed to identify key new effectors that could control macrophage polarization and impact the development of cancer or colitis. Following treatment with the supernatants of tumor cells, macrophages showed an upregulation in Fbxo38 expression. Subsequently, we further identified that FBXO38 promotes macrophage immunosuppressive function by upregulating the expression of M2-like genes via MAPK and IRF4 signaling without affecting M1-like macrophage polarization. Deletion of Fbxo38 in macrophages was found to block tumor development and protect against DSS-induced colitis. Considering the distinct regulation of tumor development by FBXO38 in T cells and macrophages, we suggest that a comprehensive understanding of FBXO38 function in different cell types is critical for its further translational usage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The RNA sequencing data (GSE243182) and single-cell sequence analysis data [20, 21] (GSE125527, GSE140228) were deposited in the Gene Expression Omnibus. All other remaining data are available within the article and Supplementary Files or can be obtained from the corresponding author (HW) upon request.
References
Vesely MD, Zhang T, Chen L. Resistance mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol. 2022;40:45–74.
Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–26.
Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Zhang J, Shi Z, Xu X, Yu Z, Mi J. The influence of microenvironment on tumor immunotherapy. FEBS J. 2019;286:4160–75.
Lin Y, Yang X, Yue W, Xu X, Li B, Zou L, et al. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell Mol Immunol. 2014;11:355–66.
Zhou X, Li W, Wang S, Zhang P, Wang Q, Xiao J, et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27:1176–89.e1175.
Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617.
Zhao X, Di Q, Liu H, Quan J, Ling J, Zhao Z, et al. MEF2C promotes M1 macrophage polarization and Th1 responses. Cell Mol Immunol. 2022;19:540–53.
Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–405.
Weisser SB, Brugger HK, Voglmaier NS, McLarren KW, van Rooijen N, Sly LM. SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J Leukoc Biol. 2011;90:483–92.
Sumner CJ, d’Ydewalle C, Wooley J, Fawcett KA, Hernandez D, Gardiner AR, et al. A dominant mutation in FBXO38 causes distal spinal muscular atrophy with calf predominance. Am J Hum Genet. 2013;93:976–83.
Akcimen F, Vural A, Durmus H, Cakar A, Houlden H, Parman YG, et al. A novel homozygous FBXO38 variant causes an early-onset distal hereditary motor neuronopathy type IID. J Hum Genet. 2019;64:1141–4.
Saferali A, Yun JH, Parker MM, Sakornsakolpat P, Chase RP, Lamb A, et al. Analysis of genetically driven alternative splicing identifies FBXO38 as a novel COPD susceptibility gene. PLoS Genet. 2019;15:e1008229.
Shang D, Dong L, Zeng L, Yang R, Xu J, Wu Y, et al. Two-stage comprehensive evaluation of genetic susceptibility of common variants in FBXO38, AP3B2 and WHAMM to severe chronic periodontitis. Sci Rep. 2015;5:17882.
Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564:130–5.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9.
Wang Z, Hao C, Zhuang Q, Zhan B, Sun X, Huang J, et al. Excretory/secretory products from trichinella spiralis adult worms attenuated DSS-induced colitis in mice by driving PD-1-mediated M2 macrophage polarization. Front Immunol. 2020;11:563784.
Zheng X, Xiao J, Jiang Q, Zheng L, Liu C, Dong C, et al. AKT2 reduces IFNbeta1 production to modulate antiviral responses and systemic lupus erythematosus. EMBO J. 2022;41:e108016.
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell. 2019;179:829-45.e20.
Boland BS, He Z, Tsai MS, Olvera JG, Omilusik KD, Duong HG, et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci Immunol. 2020;5:eabb4432.
Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–44.
Antonsen KW, Hviid CVB, Hagensen MK, Sørensen BS, Møller HJ. Soluble PD-1 (sPD-1) is expressed in human macrophages. Cell Immunol. 2021;369:104435.
Grzywa TM, Sosnowska A, Matryba P, Rydzynska Z, Jasinski M, Nowis D, et al. Myeloid cell-derived arginase in cancer immune response. Front Immunol. 2020;11:938.
Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2:194–9.
Zhang X, Fan L, Wu J, Xu H, Leung WY, Fu K, et al. Macrophage p38alpha promotes nutritional steatohepatitis through M1 polarization. J Hepatol. 2019;71:163–74.
Cheng Y, Zhu Y, Xu J, Yang M, Chen P, Xu W, et al. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol Cancer. 2018;17:13.
Jimenez-Garcia L, Herranz S, Luque A, Hortelano S. Critical role of p38 MAPK in IL-4-induced alternative activation of peritoneal macrophages. Eur J Immunol. 2015;45:273–86.
Alam MS, Gaida MM, Ogawa Y, Kolios AG, Lasitschka F, Ashwell JD. Counter-regulation of T cell effector function by differentially activated p38. J Exp Med. 2014;211:1257–70.
Acknowledgements
We thank Hangzhou Institute for Advanced Study and Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, University of Chinese Academy of Sciences. This work was supported by grants from the National Natural Science Foundation of China (81825011, 32221002, 81930038 and 82303154), the Ministry of Science and Technology of China (2018YFA0800702), the Science and Technology Commission of Shanghai Municipality (22JC1403001, HS2021SHZX001) and the China Postdoctoral Science Foundation (2022M723141).
Author information
Authors and Affiliations
Contributions
XZ and QJ performed the majority of the experiments and analyzed the data. MH, FY, MW, YQ, JW, MG and FH helped with the experiments. XZ, QJ and HW designed the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, X., Jiang, Q., Han, M. et al. FBXO38 regulates macrophage polarization to control the development of cancer and colitis. Cell Mol Immunol 20, 1367–1378 (2023). https://doi.org/10.1038/s41423-023-01081-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-023-01081-2