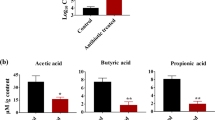
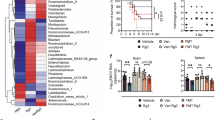
Abstract
The gut microbiome is recognized as a key modulator of sepsis development. However, the contribution of the gut mycobiome to sepsis development is still not fully understood. Here, we demonstrated that the level of Candida albicans was markedly decreased in patients with bacterial sepsis, and the supernatant of Candida albicans culture significantly decreased the bacterial load and improved sepsis symptoms in both cecum ligation and puncture (CLP)-challenged mice and Escherichia coli-challenged pigs. Integrative metabolomics and the genetic engineering of fungi revealed that Candida albicans-derived phenylpyruvate (PPA) enhanced the bactericidal activity of macrophages and reduced organ damage during sepsis. Mechanistically, PPA directly binds to sirtuin 2 (SIRT2) and increases reactive oxygen species (ROS) production for eventual bacterial clearance. Importantly, PPA enhanced the bacterial clearance capacity of macrophages in sepsis patients and was inversely correlated with the severity of sepsis in patients. Our findings highlight the crucial contribution of commensal fungi to bacterial disease modulation and expand our understanding of the host-mycobiome interaction during sepsis development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10.
Cavaillon JM, Singer M, Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med. 2020;12:e10128.
Busani S, Serafini G, Mantovani E, Venturelli C, Giannella M, Viale P, et al. Mortality in patients with septic shock by multidrug resistant bacteria: risk factors and impact of sepsis treatments. J Intensive Care Med. 2019;34:48–54.
Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614.
Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–29.
Rabani R, Volchuk A, Jerkic M, Ormesher L, Garces-Ramirez L, Canton J, et al. Mesenchymal stem cells enhance Nox2-dependent reactive oxygen species production and bacterial killing in macrophages during sepsis. Eur Respir J. 2018;51:1702021.
Liu W, Wu H, Chen L, Wen Y, Kong X, Gao WQ. Park7 interacts with p47(phox) to direct NADPH oxidase-dependent ROS production and protect against sepsis. Cell Res. 2015;25:691–706.
Eskandarian HA, Impens F, Nahori MA, Soubigou G, Coppee JY, Cossart P, et al. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science. 2013;341:1238858.
Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX, et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014;33:1304–20.
Ciarlo E, Heinonen T, Theroude C, Herderschee J, Mombelli M, Lugrin J, et al. Sirtuin 2 deficiency increases bacterial phagocytosis by macrophages and protects from chronic staphylococcal infection. Front Immunol. 2017;8:1037.
Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022;18:544–58.
Chauhan V, Kanwar SS. Lipopeptide(s) associated with human microbiome as potent cancer drug. Semin Cancer Biol. 2021;70:128–33.
Malka O, Kalson D, Yaniv K, Shafir R, Rajendran M, Ben-David O, et al. Cross-kingdom inhibition of bacterial virulence and communication by probiotic yeast metabolites. Microbiome. 2021;9:70.
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12:578386.
Gong S, Lan T, Zeng L, Luo H, Yang X, Li N, et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol. 2018;69:51–59.
Terciolo C, Dapoigny M, Andre F. Beneficial effects of Saccharomyces boulardii CNCM i-745 on clinical disorders associated with intestinal barrier disruption. Clin Exp Gastroenterol. 2019;12:67–82.
Wu G, Zhao H, Li C, Rajapakse MP, Wong WC, Xu J, et al. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet. 2015;11:e1005614.
Shao TY, Haslam DB, Bennett RJ, Way SS. Friendly fungi: symbiosis with commensal Candida albicans. Trends Immunol. 2022;43:706–17.
Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol. 2020;72:391–400.
Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Investig. 2017;127:2829–41.
Mogilnicka I, Ufnal M. Gut mycobiota and fungal metabolites in human homeostasis. Curr Drug Targets. 2019;20:232–40.
Mendoza SR, Zamith-Miranda D, Takacs T, Gacser A, Nosanchuk JD, Guimaraes AJ. Complex and controversial roles of eicosanoids in fungal pathogenesis. J Fungi. 2021;7:254.
Panpetch W, Somboonna N, Bulan DE, Issara-Amphorn J, Worasilchai N, Finkelman M, et al. Gastrointestinal colonization of Candida albicans increases serum (1–>3)-beta-d-glucan, without candidemia, and worsens cecal ligation and puncture sepsis in murine model. Shock. 2018;49:62–70.
Wagener J, MacCallum DM, Brown GD, Gow NA. Candida albicans chitin increases arginase-1 activity in human macrophages, with an impact on macrophage antimicrobial functions. mBio. 2017;8:e01820–16.
Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957–63.
Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, et al. The gut mycobiome of the human microbiome project healthy cohort. Microbiome. 2017;5:153.
Amatullah H, Shan Y, Beauchamp BL, Gali PL, Gupta S, Maron-Gutierrez T, et al. DJ-1/park7 impairs bacterial clearance in sepsis. Am J Respir Crit Care Med. 2017;195:889–905.
Rumpf T, Schiedel M, Karaman B, Roessler C, North BJ, Lehotzky A, et al. Selective SIRT2 inhibition by ligand-induced rearrangement of the active site. Nat Commun. 2015;6:6263.
Hou J, Chen Q, Wu X, Zhao D, Reuveni H, Licht T, et al. S1pr3 signaling drives bacterial killing and is required for survival in bacterial sepsis. Am J Respir Crit Care Med. 2017;196:1559–70.
Pizzolla A, Hultqvist M, Nilson B, Grimm MJ, Eneljung T, Jonsson IM, et al. Reactive oxygen species produced by the nadph oxidase 2 complex in monocytes protect mice from bacterial infections. J Immunol. 2012;188:5003–11.
Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, et al. Enhancing Nrf2 pathway by disruption of keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med. 2011;184:928–38.
Lang S, Duan Y, Liu J, Torralba MG, Kuelbs C, Ventura-Cots M, et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–38.
Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14:405–16.
Schneider SM, Girard-Pipau F, Filippi J, Hebuterne X, Moyse D, Hinojosa GC, et al. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J Gastroenterol. 2005;11:6165–69.
Jiang TT, Shao TY, Ang W, Kinder JM, Turner LH, Pham G, et al. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe. 2017;22:809–16.
Quintin J, Saeed S, Martens J, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–32.
Lam S, Bai X, Shkoporov AN, Park H, Wu X, Lan P, et al. Roles of the gut virome and mycobiome in faecal microbiota transplantation. Lancet Gastroenterol Hepatol. 2022;7:472–84.
Tso G, Reales-Calderon JA, Tan A, Sem X, Le GTT, Tan TG, et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science. 2018;362:589–95.
Shao TY, Ang W, Jiang TT, Huang FS, Andersen H, Kinder JM, et al. Commensal Candida albicans positively calibrates systemic th17 immunological responses. Cell Host Microbe. 2019;25:404–17.
Cugini C, Calfee MW, Farrow JR, Morales DK, Pesci EC, Hogan DA. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol Microbiol. 2007;65:896–906.
Coyte KZ, Schluter J, Foster KR. The ecology of the microbiome: networks, competition, and stability. Science. 2015;350:663–66.
Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic lactobacillus species in vaginal health. Res Microbiol. 2017;168:782–92.
Cabral DJ, Penumutchu S, Norris C, Morones-Ramirez JR, Belenky P. Microbial competition between escherichia coli and Candida albicans reveals a soluble fungicidal factor. Micro Cell. 2018;5:249–55.
Luo Z, Yu S, Zeng W, Zhou J. Comparative analysis of the chemical and biochemical synthesis of keto acids. Biotechnol Adv. 2021;47:107706.
Wang T, Liao Z, Man D, Yang J, He Z, Zeng Y, et al. Effects of phenylpyruvate on anxiety, depression and exercise capacity in C57BL/6J male mice. Acta Agric Univ Jiangxiensis. 2019;41:551–57.
van Spronsen FJ, Blau N, Harding C, Burlina A, Longo N, Bosch AM. Phenylketonuria. Nat Rev Dis Prim. 2021;7:36.
Sidorova-Darmos E, Wither RG, Shulyakova N, Fisher C, Ratnam M, Aarts M, et al. Differential expression of sirtuin family members in the developing, adult, and aged rat brain. Front Aging Neurosci. 2014;6:333.
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human sir2 ortholog, sirt2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–44.
Gomes P, Fleming OT, Cavadas C. Emerging role of sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol Sci. 2015;36:756–68.
Jing E, Gesta S, Kahn CR. Sirt2 regulates adipocyte differentiation through foxo1 acetylation/deacetylation. Cell Metab. 2007;6:105–14.
Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, et al. Sirt2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20:1256–61.
Jiang W, Wang S, Xiao M, Lin Y, Zhou L, Lei Q, et al. Acetylation regulates gluconeogenesis by promoting pepck1 degradation via recruiting the ubr5 ubiquitin ligase. Mol Cell. 2011;43:33–44.
Rosa AP, Jacques CE, Moraes TB, Wannmacher CM, Dutra AM, Dutra-Filho CS. Phenylpyruvic acid decreases glucose-6-phosphate dehydrogenase activity in rat brain. Cell Mol Neurobiol. 2012;32:1113–18.
Assouvie A, Daley-Bauer LP, Rousselet G. Growing murine bone marrow-derived macrophages. Methods Mol Biol. 2018;1784:29–33.
Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of mouse neutrophils. Curr Protoc Immunol. 2015;110:3–20.
Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36.
Wang P, Mu X, Zhao H, Li Y, Wang L, Wolfe V, et al. Administration of GDF3 into septic mice improves survival via enhancing LXRα-mediated macrophage phagocytosis. Front Immunol. 2021;12:647070.
Pai MY, Lomenick B, Hwang H, Schiestl R, McBride W, Loo JA, et al. Drug affinity responsive target stability (darts) for small-molecule target identification. Methods Mol Biol. 2015;1263:287–98.
Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundback T, Nordlund P, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9:2100–22.
Funding
This study was supported by the National Natural Science Foundation of China (32271230 and 32071124) to PC; the NIH Grant (P30DK120515) to BS; the National Natural Science Foundation of China (82270581) to YC; the National Key R&D Project of China (2018YFC0115301), the National Natural Science Foundation of China (81974070), the Shenzhen Science and Technology Program (JCYJ20210324131010027) and the Research Foundation of Shenzhen Hospital of Southern Medical University (PT2018GZR10) to WG.
Author information
Authors and Affiliations
Contributions
PG, RL, QY, LX, RW, FM and TC performed the experiments and analyzed the data; JL, ZZ, YH and HZ collected all the clinical data; HP, HC and YJ provided technical support; KSN revised the manuscript; WG, YC, BS, and PC designed the study, interpreted the data, drafted and edited the manuscript, and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, P., Liu, R., Yang, Q. et al. A metabolite from commensal Candida albicans enhances the bactericidal activity of macrophages and protects against sepsis. Cell Mol Immunol 20, 1156–1170 (2023). https://doi.org/10.1038/s41423-023-01070-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-023-01070-5