Abstract
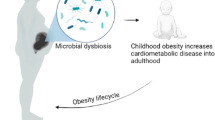
Helminth-induced Th2 immunity and gut microbiota have been recently shown to be highly effective in modulating metabolic syndromes in animal models. This study aimed to determine whether maternal immunity and microbial factors affect the induction and development of obesity in offspring. Here, Heligomosomoides polygyrus (Hp)-infected or control female C57BL/6J mice mated with normal males and their offspring were fed a high-fat diet (HFD) for 9 weeks after weaning. Our results showed that Hp-induced maternal outcomes during gestation and lactation significantly impacted offspring metabolic phenotypes. This was evidenced by results showing that offspring from helminth-infected mothers on an HFD (Hp-offspring + HFD) gained significantly less body weight than those from uninfected mothers (Cont-offspring + HFD). Hp-offspring + HFD exhibited no Th2 phenotype but displayed a pattern of gut microbiota composition similar to that of Hp-infected mothers. Cross-fostering experiments confirmed that the helminth-induced maternal attenuation of offspring obesity was mediated through both prenatal and postnatal effects. Our results further showed that helminth-infected dams and their offspring had a markedly altered gut microbiome composition, with increased production of short-chain fatty acids (SCFAs). Intriguingly, Hp-infected mothers and Hp-offspring + HFD showed increased SCFA receptor (GPR) expression in adipose and colonic tissues compared to noninfected mothers and Cont-offspring + HFD, respectively. Moreover, SCFA supplementation to the pups of uninfected control mothers during lactation protected against HFD-induced weight gain, which corresponded with changes in gut bacterial colonization. Collectively, our findings provide new insights into the complex interaction of maternal immune status and gut microbiome, Hp infection, and the immunity and gut microbiome in obese-prone offspring in infant life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13:268–73.
Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–92.
Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9.
Zaccone P, Fehérvári Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49.
Weng M, Huntley D, Huang I-F, Foye-Jackson O, Wang L, Sarkissian A, et al. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–31.
Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–32.
Harnett MM, Harnett W. Can parasitic worms cure the modern world’s ills? Trends Parasitol. 2017;33:694–705.
Su CW, Chen CY, Li Y, Long SR, Massey W, Kumar DV, et al. Helminth infection protects against high fat diet-induced obesity via induction of alternatively activated macrophages. Sci Rep. 2018;8:4607.
Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol. 2005;35:3054–63.
Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55.
Su C, Su L, Li Y, Long SR, Chang J, Zhang W, et al. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018;11:144–57.
Maizels RM, Hewitson JP, Murray J, Harcus YM, Dayer B, Filbey KJ, et al. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol. 2012;132:76–89.
Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–9.
Gaillard R, Santos S, Duijts L, Felix JF. Childhood health consequences of maternal obesity during pregnancy: a narrative review. Ann Nutr Metab. 2016;69:171–80.
Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020;367:eaaw8429.
Chen CC, Louie S, McCormick BA, Walker WA, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–83.
Chen CY, Abell AM, Moon YS, Kim KH. An advanced glycation end product (AGE)-receptor for AGEs (RAGE) axis restores adipogenic potential of senescent preadipocytes through modulation of p53 protein function. J Biol Chem. 2012;287:44498–507.
Patterson E, O’Doherty RM, Murphy EF, Wall R, O’Sullivan O, Nilaweera K, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br J Nutr. 2014;111:1905–17.
Gao M, Ma Y, Liu D. High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS ONE. 2015;10:e0119784.
Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, et al. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Investig. 2014;124:2099–112.
Shimizu I, Walsh K. The whitening of brown fat and its implications for weight management in obesity. Curr Obes Rep. 2015;4:224–9.
Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes. 2015;39:1331–8.
Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–91.
Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–9.
Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839–49.
Su CW, Chen CY, Jiao L, Long SR, Mao T, et al. Helminth-Induced and Th2-Dependent Alterations of the Gut Microbiota Attenuate Obesity Caused by High-Fat Diet. Cell Mol Gastroenterol Hepatol. 2020;10:763–78.
Trust for America’s Health and Robert Wood Johnson Foundation. The state of obesity 2018: better policies for a healthier America. https://www.tfah.org/report-details/the-state-of-obesity-2018/. Accessed December 2019.
Chang E, Hafner H, Varghese M, Griffin C, Clemente J, Islam M, et al. Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation. Sci Rep. 2019;9:16027.
Ellsworth L, Harman E, Padmanabhan V, Gregg B. Lactational programming of glucose homeostasis: a window of opportunity. Reproduction. 2018;156:R23–r42.
Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth – first 1000 days and beyond. Trends Microbiol. 2019;27:131–47.
Rook GA, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol. 2014;177:1–12.
White RA, Bjørnholt JV, Baird DD, Midtvedt T, Harris JR, Pagano M, et al. Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLoS Comput Biol. 2013;9:e1003042.
Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time?. Nutr Clin Pract. 2006;21:351–66.
Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, et al. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol. 2017;312:R74–84.
Liang X, Yang Q, Zhang L, Maricelli JW, Rodgers BD, Zhu M-J, et al. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci Rep. 2016;6:34345.
Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes. 2008;32:S32–8.
Korsmo HW, Edwards K, Dave B, Jack-Roberts C, Yu H, Saxena A, et al. Prenatal choline supplementation during high-fat feeding improves long-term blood glucose control in male mouse offspring. Nutrients. 2020;12:144.
Acknowledgements
This work was supported by grants from the National Institutes of Health-R21 AI121997 (to HNS) and R21 AI144738-01A1 (to CS) and by the Nutrition Obesity Research Center at Harvard (P30 DK040561). CS was supported by a Pilot Feasibility Grant from the Nutrition Obesity Research Center at Harvard (P30 DK040561). LJ and TM were sponsored by the China Scholarship Council. The funders had no role in the study design, collection, analysis, or interpretation of data.
Author information
Authors and Affiliations
Contributions
CS, CC, TM, NC, NS, LJ, and JL performed the experimental work and analyzed the data. CS and HNS designed the experiments, analyzed the results and wrote the paper; AF and WAW participated in editing and provided conceptual advice.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, CW., Chen, CY., Mao, T. et al. Maternal helminth infection protects offspring from high-fat-diet-induced obesity through altered microbiota and SCFAs. Cell Mol Immunol 20, 389–403 (2023). https://doi.org/10.1038/s41423-023-00979-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-023-00979-1