Abstract
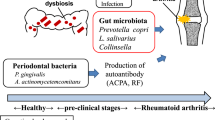
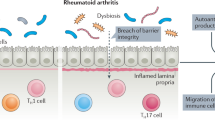
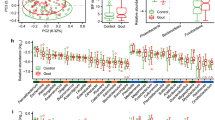
Both preclinical and established rheumatoid arthritis (RA) patients display alterations in the gut microbiome. Prevotella spp. are preferentially enriched in a subset of RA patients. Here, we isolated a Prevotella strain, P. copri RA, from the feces of RA patients and showed that colonization of P. copri RA exacerbated arthritis in a collagen-induced arthritis (CIA) model. With the presence of P. copri RA colonization, a high-fiber diet exacerbated arthritis via microbial alterations and intestinal inflammation. Colonization of P. copri together with a high-fiber diet enabled the digestion of complex fiber, which led to the overproduction of organic acids, including fumarate, succinate and short-chain fatty acids. Succinate promoted proinflammatory responses in macrophages, and supplementation with succinate exacerbated arthritis in the CIA model. Our findings highlight the importance of dysbiosis when evaluating the effects of dietary interventions on RA pathogenesis and provide new insight into dietary interventions or microbiome modifications to improve RA management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The genome sequences and RNA sequence data produced in this study are available at the Sequence Read Archive of the National Center for Biotechnology Information (the accession numbers for the sequences reported in this paper are NCBI SRA: SRR14576269).
References
Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44:291–6. https://doi.org/10.1038/ng.1076.
Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–78. https://doi.org/10.1038/nrrheum.2011.121.
Bang SY, Lee KH, Cho SK, Lee HS, Lee KW, Bae SC. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum. 2010;62:369–77. https://doi.org/10.1002/art.27272.
Guerreiro CS, Calado A, Sousa J, Fonseca JE. Diet, microbiota, and gut permeability-the unknown triad in rheumatoid arthritis. Front Med (Lausanne). 2018;5:349. https://doi.org/10.3389/fmed.2018.00349.
Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21:895–905. https://doi.org/10.1038/nm.3914.
Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. https://doi.org/10.7554/eLife.01202.
Alpizar-Rodriguez D, Lesker TR, Gronow A, Gilbert B, Raemy E, Lamacchia C, et al. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann Rheum Dis. 2019;78:590–3. https://doi.org/10.1136/annrheumdis-2018-214514.
Jubair WK, Hendrickson JD, Severs EL, Schulz HM, Adhikari S, Ir D, et al. Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheumatol. 2018;70:1220–33. https://doi.org/10.1002/art.40490.
Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021. https://doi.org/10.1038/s41579-021-00559-y.
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 2015;22:971–82. https://doi.org/10.1016/j.cmet.2015.10.001.
Marietta EV, Murray JA, Luckey DH, Jeraldo PR, Lamba A, Patel R, et al. Suppression of inflammatory arthritis by human gut-derived prevotella histicola in humanized mice. Arthritis Rheumatol. 2016;68:2878–88. https://doi.org/10.1002/art.39785.
Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016;68:2646–61. https://doi.org/10.1002/art.39783.
De Filippis F, Pasolli E, Tett A, Tarallo S, Naccarati A, De Angelis M, et al. Distinct genetic and functional traits of human intestinal prevotella copri strains are associated with different habitual diets. Cell Host Microbe. 2019;25:444–53 e443. https://doi.org/10.1016/j.chom.2019.01.004.
Gálvez EJC, Iljazovic A, Amend L, Lesker TR, Renault T, Thiemann S, et al. Distinct polysaccharide utilization determines interspecies competition between intestinal prevotella spp. Cell Host Microbe. 2020;28:838–52 e836. https://doi.org/10.1016/j.chom.2020.09.012.
Tett A, Huang KD, Asnicar F, Fehlner-Peach H, Pasolli E, Karcher N, et al. The Prevotella copri complex comprises four distinct clades underrepresented in Westernized populations. Cell Host Microbe. 2019;26:666–79 e667. https://doi.org/10.1016/j.chom.2019.08.018.
Wright DP, Rosendale DI, Robertson AM. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol Lett. 2000;190:73–9. https://doi.org/10.1111/j.1574-6968.2000.tb09265.x.
Fehlner-Peach H, Magnabosco C, Raghavan V, Scher JU, Tett A, Cox LM, et al. Distinct polysaccharide utilization profiles of human intestinal prevotella copri Isolates. Cell Host Microbe. 2019;26:680–90.e685. https://doi.org/10.1016/j.chom.2019.10.013.
De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016;24:151–7. https://doi.org/10.1016/j.cmet.2016.06.013.
Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell. 2018;175:679–94 e622. https://doi.org/10.1016/j.cell.2018.09.004.
Singh V, Yeoh BS, Walker RE, Xiao X, Saha P, Golonka RM, et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut. 2019;68:1801–1812. https://doi.org/10.1136/gutjnl-2018-316250.
Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe. 2014;16:770–7. https://doi.org/10.1016/j.chom.2014.11.003.
Connors J, Dawe N & Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. 2018;11. https://doi.org/10.3390/nu11010025.
Battino M, Forbes-Hernández TY, Gasparrini M, Afrin S, Cianciosi D, Zhang J, et al. Relevance of functional foods in the Mediterranean diet: the role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit Rev Food Sci Nutr. 2019;59:893–920. https://doi.org/10.1080/10408398.2018.1526165.
Petersson S, Philippou E, Rodomar C, Nikiphorou E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: evidence from clinical trials. Autoimmun Rev. 2018;17:1105–14. https://doi.org/10.1016/j.autrev.2018.06.007.
Hu Y, Costenbader KH, Gao X, Hu FB, Karlson EW, Lu B. Mediterranean diet and incidence of rheumatoid arthritis in women. Arthritis Care Res (Hoboken). 2015;67:597–606. https://doi.org/10.1002/acr.22481.
Ingegnoli F, Schioppo T, Scotti I, Ubiali T, De Lucia O, Murgo A, et al. Adherence to Mediterranean diet and patient perception of rheumatoid arthritis. Complement Ther Med. 2020;52:102519. https://doi.org/10.1016/j.ctim.2020.102519.
Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–53 e4114. https://doi.org/10.1016/j.cell.2021.06.019.
Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–75. https://doi.org/10.1038/nprot.2007.173.
Qiu Z, Sheridan BS. Isolating lymphocytes from the mouse small intestinal immune system. J Vis Exp. 2018. https://doi.org/10.3791/57281.
Wang Y, Li N, Yang JJ, Zhao DM, Chen B, Zhang GQ, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharm Res. 2020;157:104784. https://doi.org/10.1016/j.phrs.2020.104784.
Zheng X, Qiu Y, Zhong W, Baxter S, Su M, Li Q, et al. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics. 2013;9:818–27. https://doi.org/10.1007/s11306-013-0500-6.
Zhang H, Yohe T, Huang L, Entwistle S, Wu P, Yang Z, et al. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018;46:W95–101. https://doi.org/10.1093/nar/gky418.
Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259–69. https://doi.org/10.1016/j.immuni.2009.06.016.
Ikeda Y, Ikata T, Mishiro T, Nakano S, Ikebe M, Yasuoka S. Cathepsins B and L in synovial fluids from patients with rheumatoid arthritis and the effect of cathepsin B on the activation of pro-urokinase. J Med Invest. 2000;47:61–75.
Chiang H-I, Li J-R, Liu C-C, Liu P-Y, Chen H-H, Chen Y-M, et al. An association of gut microbiota with different phenotypes in chinese patients with rheumatoid arthritis. J Clin Med. 2019;8. https://doi.org/10.3390/jcm8111770.
Khan S, Waliullah S, Godfrey V, Khan MAW, Ramachandran RA, Cantarel BL, et al. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med. 2020;12. https://doi.org/10.1126/scitranslmed.aay6218.
Ndeh D, Rogowski A, Cartmell A, Luis AS, Baslé A, Gray J, et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 2017;544:65–70. https://doi.org/10.1038/nature21725.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1 beta through HIF-1 alpha. Nature. 2013;496:238-+. https://doi.org/10.1038/nature11986.
Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE, et al. Evidence of the immune relevance of prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:964–75. https://doi.org/10.1002/art.40003.
Zhou C, Zhao H, Xiao XY, Chen BD, Guo RJ, Wang Q, et al. Metagenomic profiling of the pro-inflammatory gut microbiota in ankylosing spondylitis. J Autoimmun. 2020;107:102360. https://doi.org/10.1016/j.jaut.2019.102360.
Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. https://doi.org/10.1111/j.1753-4887.2009.00189.x.
Slavin JL, Brauer PM, Marlett JA. Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. J Nutr. 1981;111:287–97. https://doi.org/10.1093/jn/111.2.287.
Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–31. https://doi.org/10.1038/nrmicro1817.
Nunns GR, Vigneshwar N, Kelher MR, Stettler GR, Gera L, Reisz JA, et al. Succinate activation of SUCNR1 predisposes severely injured patients to neutrophil-mediated ARDS. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004644.
Littlewood-Evans A, Sarret S, Apfel V, Loesle P, Dawson J, Zhang J, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213:1655–62. https://doi.org/10.1084/jem.20160061.
Chen Y, Ma C, Liu L, He J, Zhu C, Zheng F, et al. Analysis of gut microbiota and metabolites in patients with rheumatoid arthritis and identification of potential biomarkers. Aging (Albany NY). 2021;13. https://doi.org/10.18632/aging.203641.
Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338–56.e5321. https://doi.org/10.1016/j.cell.2021.09.019.
Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, et al. A. muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-Like TAMs. Cancer Immunol Res. 2021;9:1111–24. https://doi.org/10.1158/2326-6066.CIR-20-1019.
Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364:1179–84. https://doi.org/10.1126/science.aaw7479.
Chen BD, Jia XM, Xu JY, Zhao LD, Ji JY, Wu BX, et al. An autoimmunogenic and proinflammatory profile defined by the gut microbiota of patients with untreated systemic lupus erythematosus. Arthritis Rheumatol. 2021;73:232–43. https://doi.org/10.1002/art.41511.
Nguyen Y, Salliot C, Gelot A, Gambaretti J, Mariette X, Boutron-Ruault MC, et al. Mediterranean diet and risk of rheumatoid arthritis: findings from the French E3N-EPIC cohort study. Arthritis Rheumatol. 2021;73:69–77. https://doi.org/10.1002/art.41487.
Acknowledgements
We thank all donors who participated in this study.
Funding
This work was supported by the National Natural Science Foundation of China (81788101, 82230060, 81630064, and 81701624), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-017, 2021-I2M-1-047, 2021-I2M-1-040, and 2021-I2M-1-016), the Capital’s Funds for Health Improvement and Research (2020-2-4019) and the National Key Research and Development Program of China (Grant no. 2018YFE0207300).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: LJJ, ZHL, and XZ; implementation of in vivo and in vitro experiments: LJJ, MMS and SNY; analysis of 16 S rRNA sequencing data and the transcriptome data analysis: LJJ; statistical analysis: MMS and HZ; critical comments: YDL, YZZ, MW, TTW, XZ and ZHL; drafting of manuscript: LJJ and SNY; final approval of the version of the article to be published: all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All studies reported here were approved by the Institutional Review Board and Animal Care and Use Committee of Peking Union Medical College Hospital (PUMCH).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, L., Shang, M., Yu, S. et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell Mol Immunol 19, 1414–1424 (2022). https://doi.org/10.1038/s41423-022-00934-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-022-00934-6
Keywords
This article is cited by
-
Gold nanoparticles exhibit anti-osteoarthritic effects via modulating interaction of the “microbiota-gut-joint” axis
Journal of Nanobiotechnology (2024)
-
Regulation of polysaccharide in Wu‐tou decoction on intestinal microflora and pharmacokinetics of small molecular compounds in AIA rats
Chinese Medicine (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Microbial Mechanisms of Rheumatoid Arthritis Pathogenesis
Current Rheumatology Reports (2024)
-
The multi-kingdom microbiome of the goat gastrointestinal tract
Microbiome (2023)