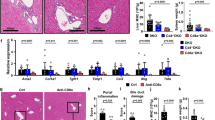
Abstract
Regulatory T cells (Treg cells) are crucial for maintaining immune tolerance. Compromising the regulatory function of Treg cells can lead to autoimmune liver disease. However, how Treg cell function is regulated has not been fully clarified. Here, we report that mice with AMP-activated protein kinase alpha 1 (AMPKα1) globally knocked out spontaneously develop immune-mediated liver injury, with massive lymphocyte infiltration in the liver, elevated serum alanine aminotransferase levels, and greater production of autoantibodies. Both transplantation of wild-type bone marrow and adoptive transfer of wild-type Treg cells can prevent liver injury in AMPKα1-KO mice. In addition, Treg cell-specific AMPKα1-KO mice display histological features similar to those associated with autoimmune liver disease, greater production of autoantibodies, and hyperactivation of CD4+ T cells. AMPKα1 deficiency significantly impairs Treg cell suppressive function but does not affect Treg cell differentiation or proliferation. Furthermore, AMPK is activated upon T cell receptor (TCR) stimulation, which triggers Foxp3 phosphorylation, suppressing Foxp3 ubiquitination and proteasomal degradation. Importantly, the frequency of Treg cells and the phosphorylation levels of AMPK at T172 in circulating blood are significantly lower in patients with autoimmune liver diseases. Conclusion: Our data suggest that AMPK maintains the immunosuppressive function of Treg cells and confers protection against autoimmune liver disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66.
Trivedi PJ, Adams DH. Mucosal immunity in liver autoimmunity: a comprehensive review. J Autoimmun. 2013;46:97–111.
Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Regulatory T cells: mechanisms of suppression and impairment in autoimmune liver disease. IUBMB Life. 2015;67:88–97.
Lapierre P, Lamarre A. Regulatory T cells in autoimmune and viral chronic hepatitis. J Immunol Res. 2015;2015:479703.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500.
Liberal R, Grant CR, Yuksel M, Graham J, Kalbasi A, Ma Y, Heneghan MA, et al. Regulatory T-cell conditioning endows activated effector T cells with suppressor function in autoimmune hepatitis/autoimmune sclerosing cholangitis. Hepatology. 2017;66:1570–84.
Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–7.
Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, Hussain MJ, et al. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999–1007.
Longhi MS, Mitry RR, Samyn M, Scalori A, Hussain MJ, Quaglia A, Mieli-Vergani G, et al. Vigorous activation of monocytes in juvenile autoimmune liver disease escapes the control of regulatory T-cells. Hepatology. 2009;50:130–42.
Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, Mieli-Vergani G, et al. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology. 2014;59:1007–15.
Lapierre P, Beland K, Yang R, Alvarez F. Adoptive transfer of ex vivo expanded regulatory T cells in an autoimmune hepatitis murine model restores peripheral tolerance. Hepatology. 2013;57:217–27.
Hardie DG. Adenosine monophosphate-activated protein kinase: a central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb Symp Quant Biol. 2011;76:155–64.
Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85.
Ma EH, Poffenberger MC, Wong AH, Jones RG. The role of AMPK in T cell metabolism and function. Curr Opin Immunol. 2017;46:45–52.
MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–98.
Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, Yurchenko E, Raissi TC, et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41–54.
Kishton RJ, Barnes CE, Nichols AG, Cohen S, Gerriets VA, Siska PJ, Macintyre AN, et al. AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 2016;23:649–62.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303.
Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R, Li B, et al. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol. 2016;292:58–67.
Lee SY, Moon SJ, Kim EK, Seo HB, Yang EJ, Son HJ, Kim JK, et al. Metformin suppresses systemic autoimmunity in roquinsan/san mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. J Immunol. 2017;198:2661–70.
Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–8.
Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, Jinasena D, et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013;39:272–85.
Li Z, Lin F, Zhuo C, Deng G, Chen Z, Yin S, Gao Z, et al. PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J Biol Chem. 2014;289:26872–81.
van Loosdregt J, Fleskens V, Fu J, Brenkman AB, Bekker CP, Pals CE, Meerding J, et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity. 2013;39:259–71.
Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA. 2007;104:4571–6.
van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–74.
Liu Y, Wang L, Predina J, Han R, Beier UH, Wang LC, Kapoor V, et al. Inhibition of p300 impairs Foxp3(+) T regulatory cell function and promotes antitumor immunity. Nat Med. 2013;19:1173–7.
Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–8.
Morawski PA, Mehra P, Chen C, Bhatti T, Wells AD. Foxp3 protein stability is regulated by cyclin-dependent kinase 2. J Biol Chem. 2013;288:24494–502.
Deng G, Nagai Y, Xiao Y, Li Z, Dai S, Ohtani T, Banham A, et al. Pim-2 kinase influences regulatory T cell function and stability by mediating Foxp3 protein N-terminal phosphorylation. J Biol Chem. 2015;290:20211–20.
Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281–7.
Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–8.
Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, et al. Knockout of the alpha2 but not alpha1 5’-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–9.
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–35.
Bochtler P, Riedl P, Gomez I, Schirmbeck R, Reimann J. Local accumulation and activation of regulatory Foxp3+ CD4 T(R) cells accompanies the appearance of activated CD8 T cells in the liver. Hepatology. 2008;48:1954–63.
Kido M, Watanabe N, Okazaki T, Akamatsu T, Tanaka J, Saga K, Nishio A, et al. Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling. Gastroenterology. 2008;135:1333–43.
Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, Qiu D, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS ONE. 2011;6:e18909.
Yu H, Huang J, Liu Y, Ai G, Yan W, Wang X, Ning Q. IL-17 contributes to autoimmune hepatitis. J Huazhong Univ Sci Technol Med Sci. 2010;30:443–6.
Diestelhorst J, Junge N, Schlue J, Falk CS, Manns MP, Baumann U, Jaeckel E, et al. Pediatric autoimmune hepatitis shows a disproportionate decline of regulatory T cells in the liver and of IL-2 in the blood of patients undergoing therapy. PLoS ONE. 2017;12:e0181107.
Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond). 2008;32:S7–12.
Hardie DG, Carling D. The AMP-activated protein kinase-fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–73.
Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–8.
Nath N, Khan M, Rattan R, Mangalam A, Makkar RS, de Meester C, Bertrand L, et al. Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem Biophys Res Commun. 2009;386:16–20.
Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009;182:8005–14.
Wang Y, Zhou L, Li Y, Guo L, Zhou Z, Xie H, Hou Y, et al. The effects of berberine on concanavalin A-induced autoimmune hepatitis (AIH) in mice and the adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) pathway. Med Sci Monit. 2017;23:6150–61.
Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15:580–7.
van Loosdregt J, Coffer PJ. Post-translational modification networks regulating FOXP3 function. Trends Immunol. 2014;35:368–78.
Tan H, Yang K, Li Y, Shaw TI, Wang Y, Blanco DB, Wang X, et al. Integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity. 2017;46:488–503.
Funding
This study was supported in part by the following grants: NHLBI (HL079584, HL080499, HL089920, HL110488, HL128014, and HL132500), NCI (CA213022), NIA (AG047776), NSFC (31870897), and NSFC (81900387). Dr. M.H. Zou is an eminent scholar of the Georgia Research Alliance.
Author information
Authors and Affiliations
Contributions
M.-H.Z. conceived the project, H.Z., Z.L., J.A., M.Z., Y.Q. designed the experiments, carried out experiments and analyzed data, Z.L. and H.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhu, H., Liu, Z., An, J. et al. Activation of AMPKα1 is essential for regulatory T cell function and autoimmune liver disease prevention. Cell Mol Immunol 18, 2609–2617 (2021). https://doi.org/10.1038/s41423-021-00790-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-021-00790-w
Keywords
This article is cited by
-
Complex Interplay Between Metabolism and CD4+ T-Cell Activation, Differentiation, and Function: a Novel Perspective for Atherosclerosis Immunotherapy
Cardiovascular Drugs and Therapy (2023)
-
Challenges and opportunities in achieving effective regulatory T cell therapy in autoimmune liver disease
Seminars in Immunopathology (2022)