Abstract
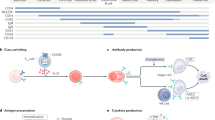
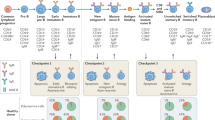
Autoimmune neurological disorders, including neuromyelitis optica spectrum disorder, anti-N-methyl-D-aspartate receptor encephalitis, anti-MOG antibody-associated disorders, and myasthenia gravis, are clearly defined by the presence of autoantibodies against neurological antigens. Although these autoantibodies have been heavily studied for their biological activities, given the heterogeneity of polyclonal patient samples, the characteristics of a single antibody cannot be definitively assigned. This review details the findings of polyclonal serum and CSF studies and then explores the advances made by single-cell technologies to the field of antibody-mediated neurological disorders. High-resolution single-cell methods have revealed abnormalities in the tolerance mechanisms of several disorders and provided further insight into the B cells responsible for autoantibody production. Ultimately, several factors, including epitope specificity and binding affinity, finely regulate the pathogenic potential of an autoantibody, and a deeper appreciation of these factors may progress the development of targeted immunotherapies for patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hozumi, N. & Tonegawa, S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc. Natl Acad. Sci. USA 73, 3628–3632 (1976).
Brack, C., Hirama, M., Lenhard-Schuller, R. & Tonegawa, S. A complete immunoglobulin gene is created by somatic recombination. Cell 15, 1–14 (1978).
Ramanathan, S. et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J. Neurol. Neurosurg. Psychiatry 89, 127–137 (2018).
Huda, S. et al. Neuromyelitis optica spectrum disorders. Clin. Med. 19, 169–176 (2019).
Ramanathan, S., Dale, R. C. & Brilot, F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun. Rev. 15, 307–324 (2016).
Gilhus, N. E. Myasthenia Gravis. N. Engl. J. Med 375, 2570–2581 (2016).
Spadaro, M. et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann. Neurol. 84, 315–328 (2018).
Reindl, M. et al. International multicenter examination of MOG antibody assays. Neurol. Neuroimmunol. Neuroinflamm. 7, e674 (2020).
Tea, F. et al. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol. Commun. 7, 145 (2019).
Mayer, M. C. et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J. Immunol. 191, 3594–3604 (2013).
Winklmeier, S. et al. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol. Neuroimmunol. Neuroinflamm. 6, 625 (2019).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Jiang, R. et al. Single-cell repertoire tracing identifies rituximab refractory B cells during myasthenia gravis relapses. Preprint at https://www.biorxiv.org/content/10.1101/840389v2.full (2019).
Wang, X., He, Y., Zhang, Q., Ren, X. & Zhang, Z. Direct comparative analysis of 10X genomics chromium and smart-seq2. Preprint at https://www.biorxiv.org/content/10.1101/615013v1 (2019).
Baran-Gale, J., Chandra, T. & Kirschner, K. Experimental design for single-cell RNA sequencing. Brief. Funct. Genomics 17, 233–239 (2018).
Tiller, T. et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 329, 112–124 (2008).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Huijbers, M. G. et al. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol. Neuroimmunol. Neuroinflamm. 6, e547 (2019).
Takata, K. et al. Characterization of pathogenic monoclonal autoantibodies derived from muscle-specific kinase myasthenia gravis patients. JCI Insight 4, e127167 (2019).
Wilson, R. et al. Condition-dependent generation of aquaporin-4 antibodies from circulating B cells in neuromyelitis optica. Brain 141, 1063–1074 (2018).
Ramberger, M. et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain 143, 1731–1744 (2020).
Sabatino, J. J. Jr., Probstel, A. K. & Zamvil, S. S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 20, 728–745 (2019).
Damato, V., Evoli, A. & Iorio, R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 73, 1342–1348 (2016).
Bennett, J. L. et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 66, 617–629 (2009).
Chihara, N. et al. Plasmablasts as migratory IgG-producing cells in the pathogenesis of neuromyelitis optica. PLoS One 8, e83036 (2013).
Chihara, N. et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc. Natl Acad. Sci. USA 108, 3701–3706 (2011).
Kim, S. H. et al. Less frequent rituximab retreatment maintains remission of neuromyelitis optica spectrum disorder, following long-term rituximab treatment. J. Neurol. Neurosurg. Psychiatry 90, 486–487 (2019).
Durozard, P. et al. Comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann. Neurol. 87, 256–266 (2020).
Cree, B. A. C. et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 394, 1352–1363 (2019).
Araki, M. et al. Clinical improvement in a patient with neuromyelitis optica following therapy with the anti-IL-6 receptor monoclonal antibody tocilizumab. Mod. Rheumatol. 23, 827–831 (2013).
Araki, M. et al. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology 82, 1302–1306 (2014).
Ayzenberg, I. et al. Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol. 70, 394–397 (2013).
Ringelstein, M. et al. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol. 72, 756–763 (2015).
Kieseier, B. C. et al. Disease amelioration with tocilizumab in a treatment-resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol. 70, 390–393 (2013).
Lauenstein, A. S., Stettner, M., Kieseier, B. C. & Lensch, E. Treating neuromyelitis optica with the interleukin-6 receptor antagonist tocilizumab. BMJ Case Rep 2014, bcr2013202939 (2014).
Trebst, C. et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J. Neurol. 261, 1–16 (2014).
Igawa, T. et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 28, 1203–1207 (2010).
Traboulsee, A. et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 19, 402–412 (2020).
Cree, B. A. et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 64, 1270–1272 (2005).
Jacob, A. et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch. Neurol. 66, 1128–1133 (2009).
Costanzi, C. et al. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology 77, 659–666 (2011).
Diaz-Manera, J. et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology 78, 189–193 (2012).
Willcox, H. N., Newsom-Davis, J. & Calder, L. R. Cell types required for anti-acetylcholine receptor antibody synthesis by cultured thymocytes and blood lymphocytes in myasthenia gravis. Clin. Exp. Immunol. 58, 97–106 (1984).
Stathopoulos, P., Kumar, A., Nowak, R. J. & O’Connor, K. C. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight 2, e94263 (2017).
Hachiya, Y. et al. Rituximab ameliorates anti-N-methyl-D-aspartate receptor encephalitis by removal of short-lived plasmablasts. J. Neuroimmunol. 265, 128–130 (2013).
Makuch, M. et al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann. Neurol. 83, 553–561 (2018).
Kreye, J. et al. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain 139, 2641–2652 (2016).
Cotzomi, E. et al. Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain 142, 1598–1615 (2019).
Lee, J. Y. et al. Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Ann. Clin. Transl. Neurol. 3, 443–454 (2016).
Meffre, E. & Wardemann, H. B-cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 20, 632–638 (2008).
Yurasov, S. et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med 201, 703–711 (2005).
Samuels, J., Ng, Y. S., Coupillaud, C., Paget, D. & Meffre, E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med 201, 1659–1667 (2005).
Stucci, S. et al. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol. Lett. 14, 5671–5680 (2017).
Suarez-Almazor, M. E., Kim, S. T., Abdel-Wahab, N. & Diab, A. Review: immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol. 69, 687–699 (2017).
Sandigursky, S. & Mor, A. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr. Rheumatol. Rep. 20, 65 (2018).
Kowarik, M. C. et al. CNS Aquaporin-4-specific B cells connect with multiple B-cell compartments in neuromyelitis optica spectrum disorder. Ann. Clin. Transl. Neurol. 4, 369–380 (2017).
Sinmaz, N. et al. Autoantibodies in movement and psychiatric disorders: updated concepts in detection methods, pathogenicity, and CNS entry. Ann. N. Y. Acad. Sci. 1351, 22–38 (2015).
Tang, X., Huang, Y., Lei, J., Luo, H. & Zhu, X. The single-cell sequencing: new developments and medical applications. Cell Biosci. 9, 53 (2019).
Ma, S., Wang, C., Mao, X. & Hao, Y. B cell dysfunction associated with aging and autoimmune diseases. Front Immunol. 10, 318 (2019).
Rubin, S. J. S., Bloom, M. S. & Robinson, W. H. B cell checkpoints in autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 15, 303–315 (2019).
Wardemann, H. & Nussenzweig, M. C. B-cell self-tolerance in humans. Adv. Immunol. 95, 83–110 (2007).
Elluru, S. R., Kaveri, S. V. & Bayry, J. The protective role of immunoglobulins in fungal infections and inflammation. Semin Immunopathol. 37, 187–197 (2015).
Nacu, A., Andersen, J. B., Lisnic, V., Owe, J. F. & Gilhus, N. E. Complicating autoimmune diseases in myasthenia gravis: a review. Autoimmunity 48, 362–368 (2015).
Chamberlain, N. et al. Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest. 126, 282–287 (2016).
Kinnunen, T. et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J. Clin. Invest. 123, 2737–2741 (2013).
Massey, J. C., Sutton, I. J., Ma, D. D. F. & Moore, J. J. Regenerating immunotolerance in multiple sclerosis with autologous hematopoietic stem cell transplant. Front. Immunol. 9, 410 (2018).
Meffre, E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann. N. Y. Acad. Sci. 1246, 1–10 (2011).
Owens, G. P. et al. Mutagenesis of the aquaporin 4 extracellular domains defines restricted binding patterns of pathogenic neuromyelitis optica IgG. J. Biol. Chem. 290, 12123–12134 (2015).
Wenke, N. K. et al. N-methyl-D-aspartate receptor dysfunction by unmutated human antibodies against the NR1 subunit. Ann. Neurol. 85, 771–776 (2019).
Takahashi, T. et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 130, 1235–1243 (2007).
Kinzel, S. & Weber, M. S. The role of peripheral CNS-directed antibodies in promoting inflammatory CNS demyelination. Brain Sci. 7, 70 (2017).
Reindl, M. & Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 15, 89–102 (2019).
Dujmovic, I. et al. Temporal dynamics of cerebrospinal fluid anti-aquaporin-4 antibodies in patients with neuromyelitis optica spectrum disorders. J. Neuroimmunol. 234, 124–130 (2011).
Kowarik, M. C. et al. The cerebrospinal fluid immunoglobulin transcriptome and proteome in neuromyelitis optica reveals central nervous system-specific B cell populations. J. Neuroinflammation 12, 19 (2015).
Fenton, K. et al. Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS One 4, e8474 (2009).
Yung, S. & Chan, T. M. Anti-DNA antibodies in the pathogenesis of lupus nephritis-the emerging mechanisms. Autoimmun. Rev. 7, 317–321 (2008).
DeGiorgio, L. A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001).
Kowal, C. et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl Acad. Sci. USA 103, 19854–19859 (2006).
Cohen-Solal, J. & Diamond, B. Lessons from an anti-DNA autoantibody. Mol. Immunol. 48, 1328–1331 (2011).
Tradtrantip, L. et al. Small-molecule inhibitors of NMO-IgG binding to aquaporin-4 reduce astrocyte cytotoxicity in neuromyelitis optica. FASEB J. 26, 2197–2208 (2012).
Tradtrantip, L. et al. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 71, 314–322 (2012).
Duan, T., Tradtrantip, L., Phuan, P. W., Bennett, J. L. & Verkman, A. S. Affinity-matured ‘aquaporumab’ anti-aquaporin-4 antibody for therapy of seropositive neuromyelitis optica spectrum disorders. Neuropharmacology 162, 107827 (2020).
Cobo-Calvo, A. et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J. Neuroinflammation 16, 134 (2019).
Hacohen, Y. et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 75, 478–487 (2018).
Malviya, M. et al. NMDAR encephalitis: passive transfer from man to mouse by a recombinant antibody. Ann. Clin. Transl. Neurol. 4, 768–783 (2017).
Gleichman, A. J., Spruce, L. A., Dalmau, J., Seeholzer, S. H. & Lynch, D. R. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J. Neurosci. 32, 11082–11094 (2012).
Kalev-Zylinska, M. L., Symes, W., Young, D. & During, M. J. Knockdown and overexpression of NR1 modulates NMDA receptor function. Mol. Cell Neurosci. 41, 383–396 (2009).
Wasterlain, C. G., Naylor, D. E., Liu, H., Niquet, J. & Baldwin, R. Trafficking of NMDA receptors during status epilepticus: therapeutic implications. Epilepsia 54, 78–80 (2013). Suppl 6.
Wright, S. et al. Epileptogenic effects of NMDAR antibodies in a passive transfer mouse model. Brain 138, 3159–3167 (2015).
Ly, L. T. et al. Affinities of human NMDA receptor autoantibodies: implications for disease mechanisms and clinical diagnostics. J. Neurol. 265, 2625–2632 (2018).
Melchers, F. Checkpoints that control B cell development. J. Clin. Invest 125, 2203–2210 (2015).
Hinson, S. R. et al. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch. Neurol. 66, 1164–1167 (2009).
Soltys, J. et al. Membrane assembly of aquaporin-4 autoantibodies regulates classical complement activation in neuromyelitis optica. J. Clin. Invest 129, 2000–2013 (2019).
Pittock, S. J. et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med 381, 614–625 (2019).
Huijbers, M. G. et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc. Natl Acad. Sci. USA 110, 20783–20788 (2013).
Koneczny, I., Cossins, J., Waters, P., Beeson, D. & Vincent, A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS One 8, e80695 (2013).
Otsuka, K. et al. Collagen Q and anti-MuSK autoantibody competitively suppress agrin/LRP4/MuSK signaling. Sci. Rep. 5, 13928 (2015).
Hughes, E. G. et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 30, 5866–5875 (2010).
Tuzun, E. & Christadoss, P. Complement associated pathogenic mechanisms in myasthenia gravis. Autoimmun. Rev. 12, 904–911 (2013).
Engel, A. G. & Fumagalli, G. Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Found Symp. 197–224 (1982).
Frenzel, A., Hust, M. & Schirrmann, T. Expression of recombinant antibodies. Front Immunol. 4, 217 (2013).
Peschke, B., Keller, C. W., Weber, P., Quast, I. & Lunemann, J. D. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 8, 646 (2017).
Quast, I. et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Invest. 125, 4160–4170 (2015).
Klooster, R. et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 135, 1081–1101 (2012).
van der Neut Kolfschoten, M. et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 (2007).
Fujihara, K. Neuromyelitis optica spectrum disorders: still evolving and broadening. Curr. Opin. Neurol. 32, 385–394 (2019).
Lennon, V. A., Kryzer, T. J., Pittock, S. J., Verkman, A. S. & Hinson, S. R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005).
Lennon, V. A. et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004).
Saadoun, S. et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349–361 (2010).
Dalmau, J. et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 18, 1045–1057 (2019).
Dalmau, J. et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 7, 1091–1098 (2008).
Dalmau, J. et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 61, 25–36 (2007).
Pruss, H. et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 75, 1735–1739 (2010).
Mikasova, L. et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 135, 1606–1621 (2012).
Moscato, E. H. et al. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 76, 108–119 (2014).
Planaguma, J. et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 138, 94–109 (2015).
Ramanathan, S., Mohammad, S. S., Brilot, F. & Dale, R. C. Autoimmune encephalitis: recent updates and emerging challenges. J. Clin. Neurosci. 21, 722–730 (2014).
Titulaer, M. J. et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 12, 157–165 (2013).
Vincent, A. ANTIBODIES AND RECEPTORS: from neuromuscular junction to central nervous system. Neuroscience 439, 48–61 (2020).
Lindstrom, J. M., Seybold, M. E., Lennon, V. A., Whittingham, S. & Duane, D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 26, 1054–1059 (1976).
Vincent, A., Palace, J. & Hilton-Jones, D. Myasthenia gravis. Lancet 357, 2122–2128 (2001).
Nowak, R. J., Dicapua, D. B., Zebardast, N. & Goldstein, J. M. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther. Adv. Neurol. Disord. 4, 259–266 (2011).
McLaughlin, K. A. et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J. Immunol. 183, 4067–4076 (2009).
O’Connor, K. C. et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 13, 211–217 (2007).
Brilot, F. et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann. Neurol. 66, 833–842 (2009).
Ramanathan, S., Al-Diwani, A., Waters, P. & Irani, S. R. The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J. Neurol. 1–9 (2019).
Lopez-Chiriboga, A. S. et al. LGI1 and CASPR2 neurological autoimmunity in children. Ann. Neurol. 84, 473–480 (2018).
van Sonderen, A., Petit-Pedrol, M., Dalmau, J. & Titulaer, M. J. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat. Rev. Neurol. 13, 290–301 (2017).
Thompson, J. et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141, 348–356 (2018).
Acknowledgements
This work was supported by the Australian National Health and Medical Research Council [APP1078643 and APP1183968] (NHRMC, Australia), Multiple Sclerosis Research Australia, and a Sydney Research Excellence Initiative grant (University of Sydney, Australia).
Author information
Authors and Affiliations
Contributions
A.Z. and F.B. designed the study. A.Z. wrote the first draft of the manuscript and prepared the figures and tables. A.Z., S.R., R.C.D., and F.B. reviewed the draft before submission.
Corresponding author
Ethics declarations
Competing interests
A.Z. reports funding from the University of Sydney Postgraduate Award (UPA, Australia) and declares no other competing interests. S.R. is a consultant on an advisory board for UCB on the treatment of MOG antibody-associated demyelination. R.C.D. and F.B. have received honoraria from Biogen Idec and Merck Serono as invited speakers.
Rights and permissions
About this article
Cite this article
Zou, A., Ramanathan, S., Dale, R.C. et al. Single-cell approaches to investigate B cells and antibodies in autoimmune neurological disorders. Cell Mol Immunol 18, 294–306 (2021). https://doi.org/10.1038/s41423-020-0510-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-0510-z
Keywords
This article is cited by
-
Advances in single-cell sequencing: insights from organ transplantation
Military Medical Research (2021)
-
Autoantibodies in neurological disease
Nature Reviews Immunology (2021)