Abstract
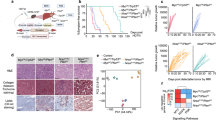
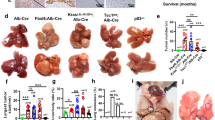
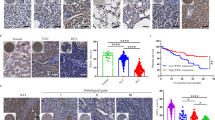
The liver is an immunologically tolerant organ and a common metastatic site of multiple cancer types. Although a role for cancer cell invasion programs has been well characterized, whether and how liver-intrinsic factors drive metastatic spread is incompletely understood. Here, we show that aberrantly activated hepatocyte-intrinsic cell cycle-related kinase (CCRK) signaling in chronic liver diseases is critical for cancer metastasis by reprogramming an immunosuppressive microenvironment. Using an inducible liver-specific transgenic model, we found that CCRK overexpression dramatically increased both B16F10 melanoma and MC38 colorectal cancer (CRC) metastasis to the liver, which was highly infiltrated by polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSCs) and lacking natural killer T (NKT) cells. Depletion of PMN-MDSCs in CCRK transgenic mice restored NKT cell levels and their interferon gamma production and reduced liver metastasis to 2.7% and 0.7% (metastatic tumor weights) in the melanoma and CRC models, respectively. Mechanistically, CCRK activated nuclear factor-kappa B (NF-κB) signaling to increase the PMN-MDSC-trafficking chemokine C-X-C motif ligand 1 (CXCL1), which was positively correlated with liver-infiltrating PMN-MDSC levels in CCRK transgenic mice. Accordingly, CRC liver metastasis patients exhibited hyperactivation of hepatic CCRK/NF-κB/CXCL1 signaling, which was associated with accumulation of PMN-MDSCs and paucity of NKT cells compared to healthy liver transplantation donors. In summary, this study demonstrates that immunosuppressive reprogramming by hepatic CCRK signaling undermines antimetastatic immunosurveillance. Our findings offer new mechanistic insights and therapeutic targets for liver metastasis intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Disibio, G. & French, S. W. Metastatic patterns of cancers: results from a large autopsy study. Arch. Pathol. Lab. Med. 132, 931–939 (2008).
Riihimaki, M., Hemminki, A., Sundquist, J. & Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 6, 29765 (2016).
Tas, F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J. Oncol. 2012, 647684 (2012).
Brodt, P. Role of the microenvironment in liver metastasis: from pre- to prometastatic niches. Clin. Cancer Res. 22, 5971–5982 (2016).
Eggert, T. & Greten, T. F. Tumor regulation of the tissue environment in the liver. Pharmacol. Ther. 173, 47–57 (2017).
Robinson, M. W., Harmon, C. & O'Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 13, 267–276 (2016).
Williamson, T., Sultanpuram, N. & Sendi, H. The role of liver microenvironment in hepatic metastasis. Clin. Transl. Med. 8, 21 (2019).
Kondo, T. et al. The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br. J. Cancer 115, 34–39 (2016).
Wu, W. et al. Fatty liver is a risk factor for liver metastasis in Chinese patients with non-small cell lung cancer. PeerJ 7, e6612 (2019).
Lee, J. W. et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567, 249–252 (2019).
Condamine, T., Ramachandran, I., Youn, J. I. & Gabrilovich, D. I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 66, 97–110 (2015).
Veglia, F., Perego, M. & Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119 (2018).
Zhou, J. et al. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut 67, 931–944 (2018).
Sun, H. et al. An inflammatory-CCRK circuitry drives mTORC1-dependent metabolic and immunosuppressive reprogramming in obesity-associated hepatocellular carcinoma. Nat. Commun. 9, 5214 (2018).
Liu, M. et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut 69, 365–379 (2020).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 (2009).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Feng, H. et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor-dependent hepatocarcinogenesis. J. Clin. Investig. 121, 3159–3175 (2011).
Yu, Z. et al. Cell cycle-related kinase mediates viral-host signalling to promote hepatitis B virus-associated hepatocarcinogenesis. Gut 63, 1793–1804 (2014).
Feng, H. et al. A CCRK-EZH2 epigenetic circuitry drives hepatocarcinogenesis and associates with tumor recurrence and poor survival of patients. J. Hepatol. 62, 1100–1111 (2015).
Mok, M. T. et al. CCRK is a novel signalling hub exploitable in cancer immunotherapy. Pharmacol. Ther. 186, 138–151 (2018).
Lupu, F. I., Burnett, J. B. & Eggenschwiler, J. T. Cell cycle-related kinase regulates mammalian eye development through positive and negative regulation of the Hedgehog pathway. Dev. Biol. 434, 24–35 (2018).
Mumert, M. et al. Functional genomics identifies drivers of medulloblastoma dissemination. Cancer Res. 72, 4944–4953 (2012).
Zhu, J. et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat. Commun. 8, 1404 (2017).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, https://doi.org/10.1126/science.aan5931 (2018).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
Lu, Z. et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature, https://doi.org/10.1038/s41586-020-2054-x (2020).
Burke, S. J. et al. NF-kappaB and STAT1 control CXCL1 and CXCL2 gene transcription. Am. J. Physiol. Endocrinol. Metab. 306, E131–E149 (2014).
Bergenfelz, C., Roxa, A., Mehmeti, M., Leandersson, K. & Larsson, A. M. Clinical relevance of systemic monocytic-MDSCs in patients with metastatic breast cancer. Cancer Immunol. Immunother. 69, 435–448 (2020).
Weide, B. et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin. Cancer Res. 20, 1601–1609 (2014).
Lee, J. W. & Beatty, G. L. Inflammatory networks cultivate cancer cell metastasis to the liver. Cell Cycle 19, 642–651 (2020).
Heymann, F. & Tacke, F. Immunology in the liver-from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 (2016).
Motohashi, S. et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J. Immunol. 182, 2492–2501 (2009).
Fujii, S. et al. NKT cells as an ideal anti-tumor immunotherapeutic. Front. Immunol. 4, 409 (2013).
Li, Z., Wu, Y., Wang, C. & Zhang, M. Mouse CD8(+)NKT-like cells exert dual cytotoxicity against mouse tumor cells and myeloid-derived suppressor cells. Cancer Immunol. Immunother. 68, 1303–1315 (2019).
De Santo, C. et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J. Clin. Investig. 118, 4036–4048 (2008).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Marshall, E. A. et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol. Cancer 15, 67 (2016).
Zhang, H. et al. Critical role of myeloid-derived suppressor cells in tumor-induced liver immune suppression through inhibition of NKT cell function. Front. Immunol. 8, 129 (2017).
Milette, S., Sicklick, J. K., Lowy, A. M. & Brodt, P. Molecular pathways: targeting the microenvironment of liver metastases. Clin. Cancer Res. 23, 6390–6399 (2017).
Acknowledgements
We acknowledge Prof. Andrew M.L. Chan for his kind gift of the B16F10 melanoma cell line. This project is supported by the University Grants Committee through the Collaborative Research Fund (C4045-18W), Theme-based Research Scheme (T11-706/18-N), General Research Fund (14108219, 14105419), the Li Ka Shing Foundation and the Terry Fox Foundation.
Author information
Authors and Affiliations
Contributions
Study concept and design: X.Z., J.Z., and A.S.L.C.; acquisition of data: X.Z., Z.X., H.S., W.Y., M.T.S.M., J.W., J.L., M.L., W.T., Y.F., H.K.S.W., S.W.T., and K.L.C.; analysis and interpretation of the data: X.Z., J.Z., and A.S.L.C.; acquisition of patient specimens: H.S., P.C.Y., J.W., P.B.S.L., A.W.H.C., K.F.T., and Q.X.; drafting of the manuscript: X.Z., J.Z., and A.S.L.C.; critical revision of the manuscript: J.Z., K.L.C., A.W.H.C., K.F.T., S.L.C., J.X., X.C., J.Y., S.P., J.J.Y.S., M.K., and A.S.L.C.; obtained funding: J.Z., J.J.Y.S., M.K., and A.S.L.C.; and study supervision: J.Z. and A.S.L.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zeng, X., Zhou, J., Xiong, Z. et al. Cell cycle-related kinase reprograms the liver immune microenvironment to promote cancer metastasis. Cell Mol Immunol 18, 1005–1015 (2021). https://doi.org/10.1038/s41423-020-00534-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-00534-2
Keywords
This article is cited by
-
T-cell infiltration and its regulatory mechanisms in cancers: insights at single-cell resolution
Journal of Experimental & Clinical Cancer Research (2024)
-
NF-κB in biology and targeted therapy: new insights and translational implications
Signal Transduction and Targeted Therapy (2024)
-
Bioinformatics-based screening and analysis of the key genes involved in the influence of antiangiogenesis on myeloid-derived suppressor cells and their effects on the immune microenvironment
Medical Oncology (2024)
-
Liver metastasis from colorectal cancer: pathogenetic development, immune landscape of the tumour microenvironment and therapeutic approaches
Journal of Experimental & Clinical Cancer Research (2023)
-
Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver
Cellular & Molecular Immunology (2022)