Abstract
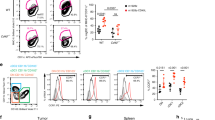
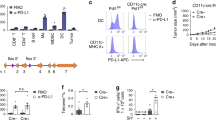
Dendritic cell (DC) tumor vaccines exert their antitumor effects through the induction of effector T cells. We recently identified Tc9 cells as a new potent antitumor effector T cell subset. However, approaches to direct DCs to preferably prime antitumor Tc9 cells should be further exploited. Here, we demonstrate that the addition of interleukin (IL)-33 potently promotes the induction of Tc9 cells by DCs in vitro and in vivo. IL-33 treatment also drives the cytotoxic activities of DC-induced Tc9 cells. Notably, IL-33 treatment enhances cell survival and proliferation of DC-primed CD8+ T cells. More importantly, the addition of IL-33 during in vitro priming of tumor-specific Tc9 cells by DCs increases the antitumor capability of Tc9 cells. Mechanistic studies demonstrated that IL-33 treatment inhibits exhaustive CD8+ T cell differentiation by inhibiting PD-1 and 2B4 expression and increasing IL-2 and CD127 (IL-7 receptor-α, IL-7Rα) expression in CD8+ T cells. Finally, the addition of IL-33 further promotes the therapeutic efficacy of DC-based tumor vaccines in the OT-I mouse model. Our study demonstrates the important role of IL-33 in DC-induced Tc9 cell differentiation and antitumor immunity and may have important clinical implications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Timmerman, J. M. & Levy, R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 50, 507–529 (1999).
Randolph, G. J., Ochando, J. & Partida-Sanchez, S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 26, 293–316 (2008).
Wang, S. et al. Dendritic cell vaccine but not idiotype-KLH protein vaccine primes therapeutic tumor-specific immunity against multiple myeloma. Front. Biosci. 12, 3566–3575 (2007).
Murphy K. A. & Griffith T. S. CD8 T cell-independent antitumor response and its potential for treatment of malignant gliomas. Cancers 8, 71 (2016).
Reiser, J. & Banerjee, A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J. Immunol. Res. 2016, 8941260 (2016).
Shrikant, P. A. et al. Regulating functional cell fates in CD8 T cells. Immunol. Res. 46, 12–22 (2010).
Ye, Z. et al. Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell. Mol. Immunol. 4, 277–285 (2007).
Yu, Y. et al. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J. Immunol. 190, 1873–1881 (2013).
Song, Y. & Yang, J. M. Role of interleukin (IL)-17 and T-helper (Th)17 cells in cancer. Biochem. Biophys. Res. Commun. 493, 1–8 (2017).
Majchrzak, K. et al. Exploiting IL-17-producing CD4+ and CD8+ T cells to improve cancer immunotherapy in the clinic. Cancer Immunol., Immunother. CII 65, 247–259 (2016).
Lu, Y. et al. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc. Natl Acad. Sci. USA 111, 2265–2270 (2014).
Dardalhon, V. et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Lu, Y., Wang, Q. & Yi, Q. Anticancer Tc9 cells: long-lived tumor-killing T cells for adoptive therapy. Oncoimmunology 3, e28542 (2014).
Liew, F. Y., Pitman, N. I. & McInnes, I. B. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 10, 103–110 (2010).
Mehraj, V., Ponte, R. & Routy, J. P. The dynamic role of the IL-33/ST2 axis in chronic viral-infections: alarming and adjuvanting the immune response. EBioMedicine 9, 37–44 (2016).
Bonilla, W. V. et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science 335, 984–989 (2012).
Villarreal, D. O. et al. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 74, 1789–1800 (2014).
Gao, K. et al. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 335, 463–471 (2013).
Gao, X. et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J. Immunol. 194, 438–445 (2015).
Kim, J. et al. Intratumorally establishing type 2 innate lymphoid cells blocks tumor growth. J. Immunol. 196, 2410–2423 (2016).
Lim, H. X., Choi, S., Cho, D. & Kim, T. S. IL-33 inhibits the differentiation and immunosuppressive activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Immunol. Cell Biol. 95, 99–107 (2017).
Dominguez, D. et al. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J. Immunol. 198, 1365–1375 (2017).
Lucarini, V. et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 6, e1317420 (2017).
Zhao, Y. et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat. Commun. 7, 12368 (2016).
Christie, D. & Zhu, J. Transcriptional regulatory networks for CD4 T cell differentiation. Curr. Top. Microbiol. Immunol. 381, 125–172 (2014).
Abu Eid, R. et al. Enhanced therapeutic efficacy and memory of tumor-specific CD8 T cells by ex vivo PI3K-delta inhibition. Cancer Res. 77, 4135–4145 (2017).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Ramadan, A. et al. Specifically differentiated T cell subset promotes tumor immunity over fatal immunity. J. Exp. Med. 214, 3577–3596 (2017).
de Kleer, I. M. et al. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 45, 1285–1298 (2016).
Xiao, X. et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 13, 981–990 (2012).
Duthie, K. A., Osborne, L. C., Foster, L. J. & Abraham, N. Proteomics analysis of interleukin (IL)-7-induced signaling effectors shows selective changes in IL-7Ralpha449F knock-in T cell progenitors. Mol. Cell. Proteom. 6, 1700–1710 (2007).
Liu, X. et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat. Med. 16, 191–197 (2010).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Bengsch, B. et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6, e1000947 (2010).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Acknowledgements
This work was supported by funds from National Natural Science Foundation of China (81372536 to S.W., 81502452 to X.C. and 81602485 to Y.Z.).
Author information
Authors and Affiliations
Contributions
S.W. and Y.Y. initiated the study. S.W. designed the experiments and wrote the paper. S.W., N.L., Y.J., J.C., H.N, Y.Z., X.C., A.W., D.W. and T.Q. performed the experiments and statistical analyses. A.W. read and edited the manuscript. Q.Y. and S.G. provided critical suggestions to this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, N., Jiang, Y., Chen, J. et al. IL-33 drives the antitumor effects of dendritic cells via the induction of Tc9 cells. Cell Mol Immunol 16, 644–651 (2019). https://doi.org/10.1038/s41423-018-0166-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0166-0
Keywords
This article is cited by
-
Discovery of highly immunogenic spleen-resident FCGR3+CD103+ cDC1s differentiated by IL-33-primed ST2+ basophils
Cellular & Molecular Immunology (2023)
-
Splenic T lymphocytes induce the formation of immunosuppressive neutrophils through IFN-γ in sepsis
Inflammation Research (2022)
-
Interleukin-37 promotes colitis-associated carcinogenesis via SIGIRR-mediated cytotoxic T cells dysfunction
Signal Transduction and Targeted Therapy (2022)