Abstract
Background
FOXL2 is a transcription factor expressed in ovarian granulosa cells. A somatic variant of FOXL2 (c.402 C > G, p.Cys134Trp) is the hallmark of adult-type granulosa cell tumours.
Methods
We generated KGN cell clones either heterozygous for this variant (MUT) or homozygous for the wild-type (WT) allele by CRISPR/Cas9 editing. They underwent RNA-Seq and bioinformatics analyses to uncover pathways impacted by deregulated genes. Cell morphology and migration were studied.
Results
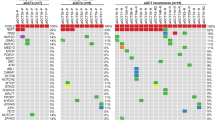
The differentially expressed genes (DEGs) between WT/MUT and WT/WT KGN cells (DEGs-WT/MUT), pointed to several dysregulated pathways, like TGF-beta pathway, cell adhesion and migration. Consistently, WT/MUT cells were rounder than WT/WT cells and displayed a different distribution of stress fibres and paxillin staining. A comparison of the DEGs-WT/MUT with those found when FOXL2 was knocked down (KD) in WT/WT KGN cells showed that most DEGs-WT/MUT cells were not so in the KD experiment, supporting a gain-of-function (GOF) scenario. MUT-FOXL2 also displayed a stronger interaction with SMAD3.
Conclusions
Our work, aiming at better understanding the GOF scenario, shows that the dysregulated genes and pathways are consistent with this idea. Besides, we propose that GOF might result from an enhanced interaction with SMAD3 that could underlie an ectopic capacity of mutated FOXL2 to bind SMAD4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study (RNA-seq) can be accessed at [Gene Expression Omnibus, GSE225781 and GSE227040] repository.
References
Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, et al. Evolution and expression of FOXL2. J Med Genet. 2002;39:916–21.
Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–81.
Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier A-C, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–42.
Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–42.
Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–66.
Shah SP, Köbel M, Senz J, Morin RD, Clarke BA, Wiegand KC, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. New Engl J Med. 2009;360:2719–29.
Hanby, A.M., walker, C. Tavassoli FA, Devilee P: Pathology and Genetics: Tumours of the Breast and Female Genital Organs. WHO Classification of Tumours series - volume IV. Lyon, France: IARC Press. Breast Cancer Res 2004;6:133. https://doi.org/10.1186/bcr788
Llano E, Todeschini AL, Felipe-Medina N, Corte-Torres MD, Condezo YB, Sanchez-Martin M, et al. The oncogenic FOXL2 C134W mutation is a key driver of granulosa cell tumors. Cancer Res. 2023;83:239–50.
Jamieson S, Fuller PJ. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Rev. 2012;33:109–44.
Jamieson S, Butzow R, Andersson N, Alexiadis M, Unkila-Kallio L, Heikinheimo M, et al. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Mod Pathol. 2010;23:1477–85.
Benayoun BA, Caburet S, Dipietromaria A, Georges A, D’Haene B, Pandaranayaka PJE, et al. Functional exploration of the adult ovarian granulosa cell tumor-associated somatic FOXL2 mutation p.Cys134Trp (c.402C>G). PLoS ONE 2010;5:e8789.
Kim J-H, Yoon S, Park M, Park H-O, Ko J-J, Lee K, et al. Differential apoptotic activities of wild-type FOXL2 and the adult-type granulosa cell tumor-associated mutant FOXL2 (C134W). Oncogene. 2011;30:1653–63.
Cheng J-C, Klausen C, Leung PCK. Overexpression of wild-type but not C134W mutant FOXL2 enhances GnRH-induced cell apoptosis by increasing GnRH receptor expression in human granulosa cell tumors. PLoS ONE. 2013;8:e55099.
Fleming NI, Knower KC, Lazarus KA, Fuller PJ, Simpson ER, Clyne CD. Aromatase is a direct target of FOXL2: C134W in granulosa cell tumors via a single highly conserved binding site in the ovarian specific promoter. PLoS ONE. 2010;5:e14389.
Rosario R, Araki H, Print CG, Shelling AN. The transcriptional targets of mutant FOXL2 in granulosa cell tumours. PLoS ONE. 2012;7:e46270.
Weis-Banke SE, Lerdrup M, Kleine-Kohlbrecher D, Mohammad F, Sidoli S, Jensen ON, et al. Mutant FOXL2C134W hijacks SMAD4 and SMAD2/3 to drive adult granulosa cell tumors. Cancer Res. 2020;80:3466–79.
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–45.
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinforma. 2013;14:128.
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–97.
Zutterling C, Todeschini A-L, Fourmy D, Busso D, Veaute X, Ducongé F, et al. The forkhead DNA-binding domain binds specific G2-rich RNA sequences. Nucleic Acids Res. 2023;51:12367–80.
Shih HP, Kopp JL, Sandhu M, Dubois CL, Seymour PA, Grapin-Botton A, et al. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. 2012;139:2488–99.
Walerych D, Lisek K, Sommaggio R, Piazza S, Ciani Y, Dalla E, et al. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat Cell Biol. 2016;18:897–909.
Kunarso G, Chia N-Y, Jeyakani J, Hwang C, Lu X, Chan Y-S, et al. Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat Genet. 2010;42:631–4.
Han H, Cho J-W, Lee S, Yun A, Kim H, Bae D, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46:D380–6.
Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14:67–79.
Liu L, Li Q, Yang L, Li Q, Du X. SMAD4 feedback activates the canonical TGF-β family signaling pathways. Int J Mol Sci. 2021;22:10024.
Rossitto M, Déjardin S, Rands CM, Le Gras S, Migale R, Rafiee M-R, et al. TRIM28-dependent SUMOylation protects the adult ovary from activation of the testicular pathway. Nat Commun. 2022;13:4412.
Penrad-Mobayed M, Perrin C, Herman L, Todeschini A-L, Nigon F, Cosson B, et al. Conventional and unconventional interactions of the transcription factor FOXL2 uncovered by a proteome-wide analysis. FASEB J. 2020;34:571–87.
Benayoun BA, Anttonen M, L’Hôte D, Bailly-Bechet M, Andersson N, Heikinheimo M, et al. Adult ovarian granulosa cell tumor transcriptomics: prevalence of FOXL2 target genes misregulation gives insights into the pathogenic mechanism of the p.Cys134Trp somatic mutation. Oncogene. 2013;32:2739–46.
Bonev B, Mendelson Cohen N, Szabo Q, Fritsch L, Papadopoulos GL, Lubling Y, et al. Multiscale 3D genome rewiring during mouse neural development. Cell. 2017;171:557–.e24.
Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–80.
Rao SSP, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–80.
Pombo A, Dillon N. Three-dimensional genome architecture: players and mechanisms. Nat Rev Mol Cell Biol. 2015;16:245–57.
Carles A, Trigo-Gonzalez G, Cao Q, Cheng S-WG, Moksa M, Bilenky M, et al. The pathognomonic FOXL2 C134W mutation alters DNA-binding specificity. Cancer Res. 2020;80:3480–91.
Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280:8343–50.
Haga K, Yamazaki M, Maruyama S, Kawaharada M, Suzuki A, Hoshikawa E, et al. Crosstalk between oral squamous cell carcinoma cells and cancer-associated fibroblasts via the TGF-β/SOX9 axis in cancer progression. Transl Oncol. 2021;14:101236.
Georges A, Auguste A, Bessière L, Vanet A, Todeschini A-L, Veitia RA. FOXL2: a central transcription factor of the ovary. J Mol Endocrinol. 2014;52:R17–33.
Nonis D, McTavish KJ, Shimasaki S. Essential but differential role of FOXL2wt and FOXL2C134W in GDF-9 stimulation of follistatin transcription in co-operation with Smad3 in the human granulosa cell line COV434. Mol Cell Endocrinol. 2013;372:42–8.
Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–9.
Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, et al. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–57.
Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, et al. Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 2005;65:9206–15.
Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA. 1995;92:1322–6.
Burns KH, Agno JE, Chen L, Haupt B, Ogbonna SC, Korach KS, et al. Sexually dimorphic roles of steroid hormone receptor signaling in gonadal tumorigenesis. Mol Endocrinol. 2003;17:2039–52.
Richards JS, Fan H-Y, Liu Z, Tsoi M, Laguë M-N, Boyer A, et al. Either Kras activation or Pten loss similarly enhance the dominant-stable CTNNB1-induced genetic program to promote granulosa cell tumor development in the ovary and testis. Oncogene. 2012;31:1504–20.
Cluzet V, Devillers MM, Petit F, Chauvin S, François CM, Giton F, et al. Aberrant granulosa cell-fate related to inactivated p53/Rb signaling contributes to granulosa cell tumors and to FOXL2 downregulation in the mouse ovary. Oncogene. 2020;39:1875–90.
Ohishi Y, Oda Y, Kurihara S, Kaku T, Kobayashi H, Wake N, et al. Nuclear localization of E-cadherin but not beta-catenin in human ovarian granulosa cell tumours and normal ovarian follicles and ovarian stroma. Histopathology. 2011;58:423–32.
Watson RH, Roy WJJ, Davis M, Hitchcock A, Campbell IG. Loss of heterozygosity at the alpha-inhibin locus on chromosome 2q is not a feature of human granulosa cell tumors. Gynecol Oncol. 1997;65:387–90.
Acknowledgements
The authors are indebted to Emma Vidal, Lakshmi Balasubramaniam, Joseph d’Alessandro and Alexandros Glentis for their help and advices.
Funding
This work was supported by the University of Paris Cité and the Centre National de la Recherche Scientifique and by ARC (Association pour la Recherche contre le Cancer) and Les Entreprises contre le cancer.
Author information
Authors and Affiliations
Contributions
LH: conceptualisation, formal analysis, investigation, methodology, validation, visualisation and writing—original draft. AA: investigation. BL: investigation. CDC: investigation. RAV: conceptualisation, formal analysis, funding acquisition, project administration, supervision, validation, writing—original draft. ALT: conceptualisation, formal analysis, funding acquisition, investigation, project administration, supervision, validation, visualisation, writing—original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herman, L., Amo, A., Legois, B. et al. A cellular model provides insights into the pathogenicity of the oncogenic FOXL2 somatic variant p.Cys134Trp. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02613-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02613-x