Abstract
Background
13–15% of breast cancer/BC patients diagnosed as pathological complete response/pCR after neoadjuvant systemic therapy/NST suffer from recurrence. This study aims to estimate the rationality of organoid forming potential/OFP for more accurate evaluation of NST efficacy.
Methods
OFPs of post-NST residual disease/RD were checked and compared with clinical approaches to estimate the recurrence risk. The phenotypes of organoids were classified via HE staining and ER, PR, HER2, Ki67 and CD133 immuno-labeling. The active growing organoids were subjected to drug sensitivity tests.
Results
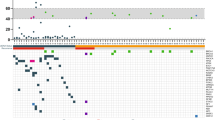
Of 62 post-NST BC specimens, 24 were classified as OFP-I with long-term active organoid growth, 19 as OFP-II with stable organoid growth within 3 weeks, and 19 as OFP-III without organoid formation. Residual tumors were overall correlated with OFP grades (P < 0.001), while 3 of the 18 patients (16.67%) pathologically diagnosed as tumor-free (ypT0N0M0) showed tumor derived-organoid formation. The disease-free survival/DFS of OFP-I cases was worse than other two groups (Log-rank P < 0.05). Organoids of OFP-I/-II groups well maintained the biological features of their parental tumors and were resistant to the drugs used in NST.
Conclusions
The OFP would be a complementary parameter to improve the evaluation accuracy of NST efficacy of breast cancers.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data generated in this study are available within the article and its supplementary data files.
References
Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62:640–7.
Heil J, Kuerer HM, Pfob A, Rauch G, Sinn HP, Golatta M, et al. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: current evidence and future challenges. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:61–71.
Spring LM, Bar Y, Isakoff SJ. The evolving role of neoadjuvant therapy for operable breast cancer. J Natl Compr Canc Netw. 2022;20:723–34.
Derouane F, van Marcke C, Berliere M, Gerday A, Fellah L, Leconte I, et al. Predictive biomarkers of response to neoadjuvant chemotherapy in breast cancer: current and future perspectives for precision medicine. Cancers (Basel). 2022;14:3876.
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13–21.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Schaefgen B, Mati M, Sinn HP, Golatta M, Stieber A, Rauch G, et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol. 2016;23:789–95.
Members of Breast Cancer Expert Panel on, C. Expert panel consensus on pathological diagnosis of breast cancer with neoadjuvant therapy, the 2020 version. Zhonghua Bing Li Xue Za Zhi. 2020;49:296–304.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (Lond, Engl). 2014;384:164–72.
Conforti F, Pala L, Sala I, Oriecuia C, De Pas T, Specchia C, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375:e066381.
Tasoulis MK, Lee HB, Yang W, Pope R, Krishnamurthy S, Kim SY, et al. Accuracy of post-neoadjuvant chemotherapy image-guided breast biopsy to predict residual cancer. JAMA Surg. 2020;155:e204103.
Goto W, Kashiwagi S, Takada K, Asano Y, Takahashi K, Fujita H, et al. Significance of intrinsic breast cancer subtypes on the long-term prognosis after neoadjuvant chemotherapy. J Transl Med. 2018;16:307.
Takaoka M, OS, Ikejiri H, Shidahara T, Miyoshi Y, Takahashi M, et al. Pathological complete response patients after neoadjuvant chemotherapy in breast cancer. Acta Med Okayama. 2022;76:105–11.
Zhou Z, Cong L, Cong X. Patient-derived organoids in precision medicine: drug screening, organoid-on-a-chip and living organoid biobank. Front Oncol. 2021;11:762184.
Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180:188–204.e122.
Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:3991.
Li M, Izpisua Belmonte JC. Organoids—preclinical models of human disease. N Engl J Med. 2019;380:569–79.
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–86.e310.
Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–97.e811.
Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauve CG, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019;25:1607–14.
Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–6.
Wang HM, Zhang CY, Peng KC, Chen ZX, Su JW, Li YF, et al. Using patient-derived organoids to predict locally advanced or metastatic lung cancer tumor response: a real-world study. Cell Rep. Med. 2023;4:100911.
Xu R, Zhou X, Wang S, Trinkle C. Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharm Ther. 2021;218:107668.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59.
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–7.
Hagenaars SC, de Groot S, Cohen D, Dekker TJA, Charehbili A, Meershoek-Klein Kranenbarg E, et al. Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer. Int J Cancer. 2021;149:1181–8.
Vasmel JE, Vreuls CPH, Manson QF, Charaghvandi RK, van Gorp J, van Leeuwen AMG, et al. Tumor-infiltrating lymphocytes in low-risk patients with breast cancer treated with single-dose preoperative partial breast irradiation. Int J Radiat Oncol Biol Phys. 2021;109:1325–31.
Nie JH, Yang T, Li H, Ye HS, Zhong GQ, Li TT, et al. Identification of GPC3 mutation and upregulation in a multidrug resistant osteosarcoma and its spheroids as therapeutic target. J Bone Oncol. 2021;30:100391.
Ye HS, Gao HF, Li H, Nie JH, Li TT, Lu MD, et al. Higher efficacy of resveratrol against advanced breast cancer organoids: a comparison with that of clinically relevant drugs. Phytother Res. 2022;36:3313–24.
Xiong L, Nie JH, Lin XM, Wu JB, Chen Z, Xu B, et al. Biological implications of PTEN upregulation and altered sodium/iodide symporter intracellular distribution in resveratrol-suppressed anaplastic thyroid cancer cells. J Cancer. 2020;11:6883–91.
Varela CL, Amaral C, Tavares da Silva E, Lopes A, Correia-da-Silva G, Carvalho RA, et al. Exemestane metabolites: synthesis, stereochemical elucidation, biochemical activity and anti-proliferative effects in a hormone-dependent breast cancer cell line. Eur J Med Chem. 2014;87:336–45.
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81.
Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105:321–33.
Loibl S, Denkert C. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1029–30.
Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J, et al. Patient-derived organoids can guide personalized-therapies for patients with advanced breast cancer. Adv Sci (Weinh). 2021;8:e2101176.
Pan B, Li X, Zhao D, Li N, Wang K, Li M, et al. Optimizing individualized treatment strategy based on breast cancer organoid model. Clin Transl Med. 2021;11:e380.
Guillen KP, Fujita M, Butterfield AJ, Scherer SD, Bailey MH, Chu Z, et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 2022;3:232–50.
Pelizzari G, Gerratana L, Basile D, Fanotto V, Bartoletti M, Liguori A, et al. Post-neoadjuvant strategies in breast cancer: From risk assessment to treatment escalation. Cancer Treat Rev. 2019;72:7–14.
Steenbruggen TG, van Seijen M, Janssen LM, van Ramshorst MS, van Werkhoven E, Vrancken Peeters M, et al. Prognostic value of residual disease after neoadjuvant therapy in HER2-positive breast cancer evaluated by residual cancer burden, neoadjuvant response Index, and Neo-Bioscore. Clin Cancer Res. 2019;25:4985–92.
Li L, Knutsdottir H, Hui K, Weiss MJ, He J, Philosophe B, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019;4:e121490.
Huang L, Bockorny B, Paul I, Akshinthala D, Frappart PO, Gandarilla O, et al. PDX-derived organoids model in vivo drug response and secrete biomarkers. JCI Insight. 2020;5:e135544.
Tiriac H, Belleau P, Engle DD, Plenker D, Deschenes A, Somerville TDD, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8:1112–29.
Tang Y, Wang T, Hu Y, Ji H, Yan B, Hu X, et al. Cutoff value of IC(50) for drug sensitivity in patient-derived tumor organoids in colorectal cancer. iScience. 2023;26:107116.
Veninga V, Voest EE. Tumor organoids: opportunities and challenges to guide precision medicine. Cancer Cell. 2021;39:1190–201.
Shiihara M, Ishikawa T, Saiki Y, Omori Y, Hirose K, Fukushige S, et al. Development of a system combining comprehensive genotyping and organoid cultures for identifying and testing genotype-oriented personalised medicine for pancreatobiliary cancers. Eur J Cancer. 2021;148:239–50.
Donzelli S, Cioce M, Sacconi A, Zanconato F, Daralioti T, Goeman F, et al. A PIK3CA-mutant breast cancer metastatic patient-derived organoid approach to evaluate alpelisib treatment for multiple secondary lesions. Mol Cancer. 2022;21:152.
Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173:515–28.e517.
Vaishampayan UN, Burger AM, Sausville EA, Heilbrun LK, Li J, Horiba MN, et al. Safety, efficacy, pharmacokinetics, and pharmacodynamics of the combination of Sorafenib and Tanespimycin. Clin Cancer Res. 2010;16:3795–804.
Acknowledgements
We thank Jian-Li He and Xuan Luo for their technical support and insightful discussions.
Funding
This work was supported by grants from the Special Fund of Foshan Summit Plan (NO. 2020B018, 2019D039, and 2019D041), the National Natural Science Foundation of China (No. 81272786 and 81450016), and the Special Fund of South China University of Technology from Central Government of China.
Author information
Authors and Affiliations
Contributions
H.L., D.Z., G.L.Y., W.L., and J.L., conceived and designed the experiments; D.Z., S.Q. Y., H.Q.H., J.J.S., and P.X.C. recruited the patients, provided the resected tissues, and collected clinical information; J.L., evaluated the pathologic outcome after neoadjuvant and organoid pathological identification; H.S.Y., J.H.N., M.D.L., T.T.L., B.L.D., and S.L. performed the experiments, analyzed the data and edited the figures; H.S.Y. wrote the manuscript; All the authors critically reviewed the manuscript. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Specimen collection and research contents were examined and approved by the Research Ethics Committee of Foshan First People’s Hospital (L [2021] NO. 9). All procedures are carried out by the Helsinki Declaration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, HS., Zhou, D., Li, H. et al. Organoid forming potential as complementary parameter for accurate evaluation of breast cancer neoadjuvant therapeutic efficacy. Br J Cancer 130, 1109–1118 (2024). https://doi.org/10.1038/s41416-024-02595-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-024-02595-w